Wang Laboratory
INQUIRIES
Xiaogang Wang, PhD
Assistant Professor, Microbiology
Center for One Health Research (COHR)
Department of Biomedical Sciences and Pathobiology
Email: xgwang@vt.edu
Phone: 540-231-5010
-
Bio Item
 Xiaogang Wang, PhD , bio
Xiaogang Wang, PhD , bioAssistant Professor, Microbiology
About the Wang Lab
We study the pathogenesis of staphylococcal infections and extracellular membrane vesicles.

Staphylococcus aureus is a Gram-positive opportunistic pathogen that causes a wide range of human and animal diseases, from mild skin lesions to invasive infections such as pneumonia, bacteremia, and endocarditis. Staphylococcal infections, particularly those caused by methicillin-resistant S. aureus (MRSA) have become a significant concern in both community and hospital settings due to their resistance to most commonly used antibiotics. Despite extensive efforts, vaccines designed to prevent S. aureus diseases have not succeeded in human trials, and much remains to be learned about how this pathogen evades host immune responses and causes disease.
Our laboratory is broadly interested in defining the critical bacterial and host factors that contribute to staphylococcal infections and elucidating the molecular mechanisms underlying
S. aureus-host interactions.

Extracellular membrane vesicles (MVs) are spherical membrane nanoparticles secreted by eukaryotes, archaea, and bacteria. MVs carry biologically active molecules, including proteins, lipids, carbohydrates, and nucleic acids. MVs were initially considered a depot for cells to dispose of unwanted cellular materials. However, recent evidence reveals that MVs may serve as a novel mechanism for cell-free intercellular communication facilitated by cellular internalization or membrane fusion and the delivery of MV cargo into recipient host cells.
As one of the earliest groups to study MVs produced by S. aureus, we have developed deep interest in the biology of vesicles. Specifically, we focus on understanding the biogenesis of S. aureus MVs and the fundamental roles that MVs play in S. aureus physiology and pathogenesis.
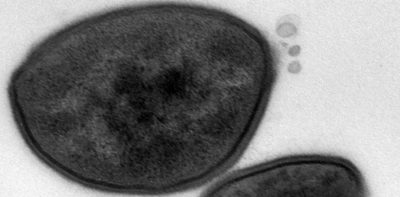
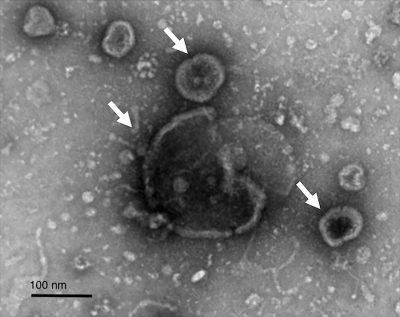
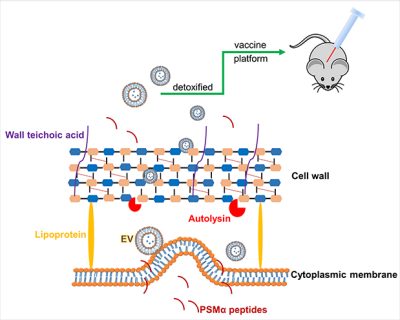
Staphylococcus aureus extracellular membrane vesicles
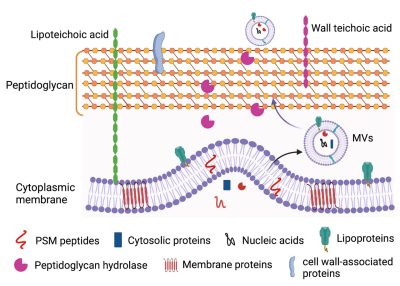
S. aureus employs a diverse array of virulence factors, both surface-associated and secreted, to colonize and invade host tissues and to evade the host immune response. Lacking sophisticated secretion systems, e.g., T3SS, T4SS, and T6SS, which transport bacterial virulence factors directly into host cells, S. aureus secretes its products into the external environment where they may be inactivated by host proteases and toxin-neutralizing antibodies. In the past decade, the production of extracellular membrane vesicles (MVs) has been characterized from various S. aureus isolates. S. aureus MVs encapsulate a wide array of bacterial components, including cytosolic, surface, and membrane proteins, as well as surface adhesins, lipoproteins, and secreted toxins and proteases. The production of MVs is notably increased in response to environmental stresses encountered during infection.
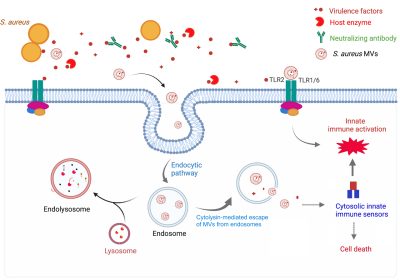
S. aureus MVs are cytolytic to multiple host cell types and elicit the production of pro-inflammatory mediators in vitro and in vivo. Moreover, S. aureus MVs are internalized into human macrophages. These findings suggest that MV production might serve as an important virulence strategy, allowing S. aureus to deliver its factors into host cells and modulate cellular functions during infection. Our laboratory is dedicated to thoroughly investigating S. aureus MVs and their roles in staphylococcal infections using a comprehensive approach that integrates molecular genetics, biochemistry, cell biology, and in vivo studies. Our studies include:
- Characterize bacterial and host factors that modulate MV biogenesis
- Evaluate the in vivo production of S. aureus MVs and their impact on the host
- Unraveling the mechanisms by which MVs and their associated factors contribute to staphylococcal infections.
Host-derived extracellular membrane vesicles
The production of extracellular membrane vesicles represents a secretory pathway common to mammalian cells, fungi, and bacteria that allows cell-free intercellular communication. Based on the size and mechanism of generation, host-derived extracellular vesicles are mainly categorized into three subtypes: exosomes, microvesicles (also called ectosomes), and apoptotic bodies. The evidence gathered from various studies has revealed that host-derived vesicles play unexpected functions in broad biological processes, including antigen presentation, inflammation, tissue homeostasis, and cancer metastasis.
Likewise, an increasing body of work has suggested that host-derived vesicles may also play a critical role in the host immune defense against pathogens, though their role in S. aureus infections remains underexplored. We have become particularly interested in the biological functions of host-derived vesicles in the context of staphylococcal infections and their potential impact on disease progression.
Translational study of extracellular membrane vesicles
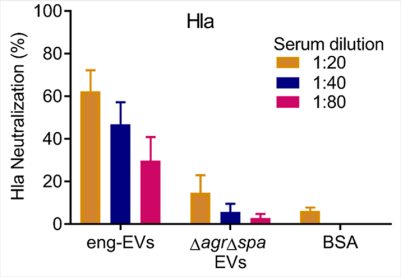
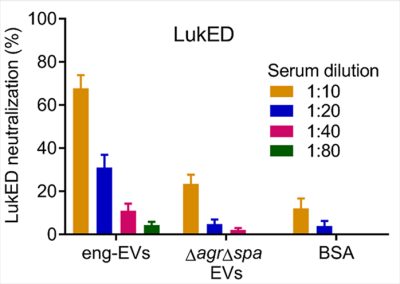
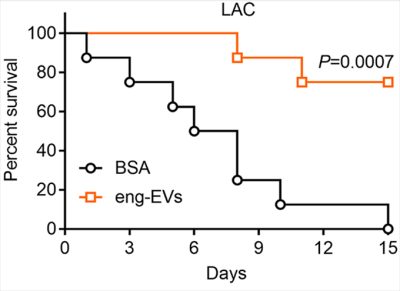
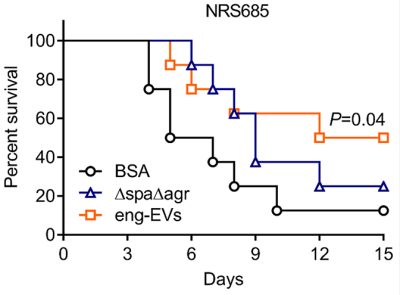
MVs are nano-sized particles that encapsulate various antigens and protect their cargo from degradation by environmental factors. Bacterial MVs possess inherent adjuvanticity and elicit antigen-specific adaptive immune responses to bacterial components. By employing genetical engineering methods, bacterial MVs can be modified to carry specific molecules and deliver them into host cells. We genetically manipulated an S. aureus agr mutant to produce nontoxic MVs for use as a vaccine platform (See figures). Engineered MVs containing detoxified pore-forming toxins elicited toxin-neutralizing antibodies and protected immunized mice against lethal sepsis. Although these studies showed potential of staphylococcal MVs to serve as a vaccine platform, MVs are produced from S. aureus in relatively low yields and the immunized animals were only partially protected in the study. In our ongoing work, we aim to employ genetic approaches to further manipulate the engineered MVs to enhance their yield and protective efficiency. Given that our lab is situated in an excellent environment for studying veterinary medicine, we are particularly interested in exploring the potential of MVs as a vaccine platform against infectious animal diseases.
Current Lab Members
Xiaogang Wang, PhD, Assistant Professor of Microbiology
Dr. Xiaogang Wang earned his Ph.D. in Microbiology from the University of Chinese Academy of Sciences in 2009. Following his doctoral studies, he pursued postdoctoral training in the University of Kansas Medical Center, University of California, Berkeley, and Harvard Medical School from 2009 to 2018. Before joining Virginia Tech in 2024, Xiaogang served as a faculty member in the Division of Infectious Diseases at Brigham and Women’s Hospital and as an instructor at Harvard Medical School. His major research interest is in the pathogenesis of staphylococcal infections, extracellular membrane vesicles, and vaccine development.
Jinger Lei, PhD Student
Jinger Lei is a first-year PhD student from China, currently working under the guidance of Dr. Wang on extracellular membrane vesicles of Staphylococcus aureus. During her undergraduate study, she developed am interest in antibiotic resistant bacteria and collateral sensitivity effects. She also gained valuable experience as a research intern in Canada, working on antimicrobial drug repurposing against MRSA. Lei is committed to advancing the understanding of bacteria that affect both human and animal health.
Research assistant:
We are seeking a highly motivated research assistant to provide research support in the area of microbial pathogenesis and animal studies. The successful candidate will work independently, perform research activities and analysis, and contribute to general maintenance of the laboratory. If you are interested in the position, please contact Xiaogang Wang directly.
PhD students:
Prospective PhD students interested in joining the lab are encouraged to contact Xiaogang via email for more information. Dr. Xiaogang Wang is an affiliated faculty member of the Infectious Disease Interdisciplinary Graduate Education Program (ID IGEP) and the Molecular and Cellular Biology (MCB) program at Virginia Tech. We particularly welcome students from these programs to apply for a rotation.
Undergraduate students:
Virginia Tech students majoring in Microbiology or similar fields are encouraged to apply for the research opportunity in the lab.
- Wang X* and Lee JC. Staphylococcus aureus extracellular vesicles: an evolving story. Trends in Microbiology. 2024, Apr 24: S0966-842X(24)00088-X (*Correspondence).
- Wang X, Uppu DSSM, Dickey SW, Burgin DL, Otto M, Lee JC. Staphylococcus aureus delta toxin plays a dual role in extracellular membrane vesicle biogenesis and amyloid formation. mBio, 2023; 14(5): e0174823
- Uppu DSSM, Wang X, Lee JC. Contribution of extracellular membrane vesicles to the secretome of Staphylococcus aureus. mBio, 2023; 14(1): e0357122
- Shibamura-Fujiogi M, Wang X, Maisat W, Koutsogiannaki S, Li Y, Chen Y, Lee JC, Yuki K. GltS as a Regulator of Biofilm Formation in Methicillin-resistant Staphylococcus aureus. Communications Biology, 2022; 5(1):1284
- Wang X, Koffi PF, English OF, Lee JC. Staphylococcus aureus extracellular vesicles: A story of toxicity and the stress of 2020. Toxins (Basel), 2021; 13(2): 75
- Wang X, Eagen WJ, Lee JC. Orchestration of human macrophage NLRP3 inflammasome activation by Staphylococcus aureus extracellular vesicles. Proceedings of the National Academy of Sciences USA, 2020; 117(6): 3174 – 3184. PMID: 31988111.
- Rausch M, Deisinger J, Ulm H, Müller A, Li W, Hardt P, Sylvester M, Wang X, Li X, Müller C, Engeser M, Vollmer W, Sahl HG, Lee JC, Schneider T. Coordination of capsule assembly and cell wall biosynthesis in Staphylococcus aureus. Nature Commmunications, 2019; 10(1): 1404.PMID: 30926919.
- Wang X, Thompson CD, Weidenmaier C, Lee JC. Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform. Nature Communications, 2018; 9(1): 1379 - Recommended by F1000Prime
- Wang X, Hybiske K, Stephens RS. Direct visualization of the expression and localization of chlamydial effector proteins within infected host cells. Pathogens and Disease, 2018; fty011. PMID: 29390129.
- Wang X*, Hybiske K., Stephens RS. Orchestration of the mammalian glucose transporter proteins-1 and 3 by Chlamydia is required for intracellular growth and infectivity. Pathogens and Disease, 2017; 75(8), ftx108. PMID: 29040458. (* Correspondence).
- Li X, Wang X, Thompson CD, Park S, Park WB, Lee JC. Preclinical efficacy of clumping factor A in prevention of Staphylococcus aureus infection. mBio, 2016; 7(1): e02232-15. Doi: 10.1128/mBio. 02232-15.
- Misawa Y, Kelley KA, Wang X, Wang LH, Park WB, Birtel J, Saslowsky D, Lee JC. Staphylococcus aureus colonization of the mouse gastrointestinal tract is modulated by wall teichoic acid, capsule, and surface proteins. PLoS Pathogens, 2015; 11(7): e1005061. doi:10.1371/journal.ppat.1005061
- Wang X, Schwarzer C, Hybiske K, Machen TE., Stephens RS. Developmental stage oxidoreductive states of Chlamydia and infected host cells. mBio, 2014; 5(6):e01924-14. doi:10.1128/mBio.01924-14.
- Gao XF, Wang X, Pham TH, Feuerbacher LA, Lubos ML, Huang MZ, Olsen R, Mushegian A, Slawson C,Hardwidge PR. NleB, a bacterial effector with glycosyltransferase activity, targets GAPDH function to inhibit NFκB activation. Cell Host & Microbe, 2013; 13: 87-99
- Wang X, Gao XF, Hardwidge PR. Heat-labile enterotoxin-induced activation of NF-κB and MAPK pathways in intestinal epithelial cells impacts enterotoxigenic Escherichia coli. Cellular Microbiology, 2012; 14: 1231-1241.
- Wang X, Hardwidge PR. Enterotoxigenic Escherichia coli prevents host NF-κB activation by targeting IkBa polyubiquitination. Infection and Immunity, 2012; 80: 4417-4425
The full publications can be found at Xiaogang Wang's Google Scholar profile.