Gaji Laboratory
About the Gaji Lab
Toxoplasmosis
Our lab is interested in studying the host-pathogen interactions of intracellular pathogens. Specifically, our work focuses on Toxoplasma gondii, a protozoan parasite that causes fatal encephalitis in immunodeficient individuals, miscarriage in pregnant women, and blindness and cognitive impairment in congenitally infected children.
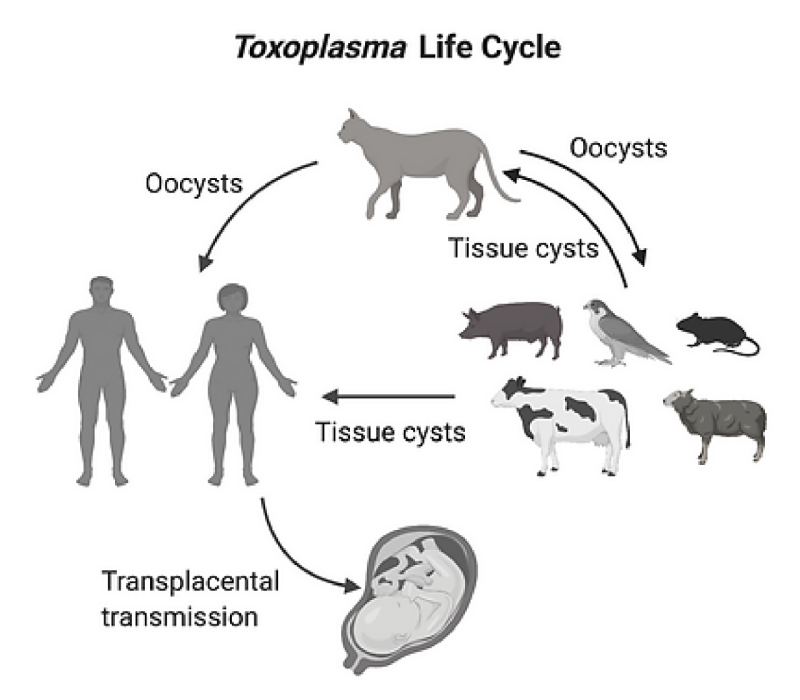
Toxoplasma has an indirect mode of life cycle where cats act as the definitive host. For Toxoplasma, the sexual phase of the life cycle occurs in intestinal epithelial cells and the parasite is then shed as oocysts in cats’ feces. These oocysts act as a source of infection to a wide number of rodents, farm animals and birds that serve as intermediate hosts for this parasite. Humans can acquire Toxoplasma infection primarily by three routes: Ingestion of food or water contaminated with Toxoplasma oocysts, ingestion of contaminated meat obtained from an infected intermediate host, or by transplacental transmission during pregnancy.
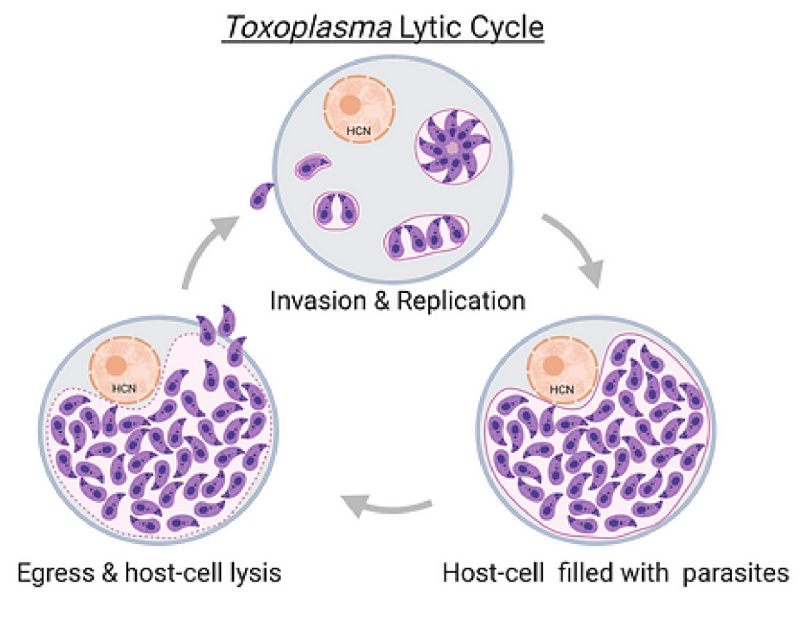
Toxoplasma is an obligatory intracellular parasite and its intracellular lifestyle begins with the parasite actively invading the host-cell. In the host-cell T. gondii is surrounded a parasitophorous vacuole within which it replicates by a process known as endodyogeny where one parasite divides into two. When there are about 64-128 parasites per host cell depending on host-cell size, newly formed daughter parasites egress resulting in destruction of the host-cell. In Toxoplasmosis, most of the pathology results due to lysis of the host cell during parasite egress process. Hence identification and characterization of unique parasite factors that are involved at any stages of the lytic cycle of Toxoplasma including invasion, replication and egress is vital in developing novel therapeutics.
TKL Kinases in Toxoplasma
Protein kinases are involved in regulating many important biological events in a cell through phosphorylation and have been exploited as drug targets in many disease contexts. In Toxoplasma also, kinases have been shown to play a key role in parasite motility, invasion, replication, egress and also survival within the host by nullifying host defense factors. An interesting set of kinases present in Toxoplasma genome are proteins that belong to Tyrosine Kinase Like (TKL) family and our long-term goal is to define the role of these kinases in Toxoplasma biology.
Bioinformatics analysis of Toxoplasma genome revealed that there are eight genes that are annotated as TKLs and six of these have been classified as being important for parasite growth in vitro. We have named the TKLs important for parasite growth numerically according to their predicted fitness scores and endogenously tagged them with an epitope tag to determine their localization in the parasite. Interestingly, these kinases localize to various compartments in the parasite including the nucleus, cytosol, IMC and Golgi.
Since many of these TKL kinases have been predicted to be important for parasite fitness, we hypothesize that these proteins play critical roles in parasite propagation in vitro and pathogenesis in vivo. Hence, we would like to address the following questions towards characterization of these proteins in Toxoplasma biology.
- What is the precise role of these proteins in the lytic cycle of Toxoplasma?
- What is the signaling network each of these kinases are involved in?
- What is the role of these proteins in acute and chronic toxoplasmosis?

Primary Investigator

Raj Gaji, BVSc, MVSc, PhD
Assistant Professor of Parasitology in the Department of Biomedical Sciences and Pathobiology
Raj completed his PhD from Gluck Equine Research Center at the University of Kentucky. During his graduate study, he trained with Dr. Dan Howe and his research was focused on the protozoan parasite Sarcocystis neurona that causes Equine Protozoal Myeloencephalitis (EPM) in horses.
He later did his postdoctoral work with Dr. Vern Carruthers (University of Michigan, Ann Arbor) and Dr. Gustavo Arrizabalaga (Indiana University School of Medicine, Indianapolis). During his postdoctoral studies, Raj studied Toxoplasma gondii, another protozoan parasite of medical and veterinary importance. Raj is currently working as an Assistant Professor in the Dept. of Biomedical Sciences and Pathobiology at Virginia Tech and his lab's research is focused on studying the role of TKL family kinases in Toxoplasma biology.
Current team members

Dima Hajj Ali, MS
Dima is a Ph.D. student in the lab. Dima completed her Masters degree from American University of Beirut, Lebanon.

Padmaja Mandadi, MS
Padmaja is a Ph.D. student in the lab. Padmaja obtained her Masters degree from India.

Sam Calvelli
Sam is a sophomore microbiology major at Virginia Tech and is currently working as our Lab Assistant.
Alumni
- Jen Nguyen - Undergraduate Research Student - Spring 2023
- Kyla Simmons - Undergraduate Research Student - Spring 2022
- Montano H, Anandkrishnan R, Carruthers, VB and Gaji RY*. (2023). TgTKL4 is a novel kinase that plays an important role in Toxoplasma morphology and fitness. mSphere. e00649-22. *Senior and corresponding author.
- Gaji RY*, Sharp A and Brown A. 2021. Protein Kinases in Toxoplasma gondii. International Journal for Parasitology. *Corresponding author.
- Varberg J, Coppens I, Arrizabalaga, G and Gaji RY*. 2018. TgTKL1 is a unique plant like nuclear kinase that plays an essential role in acute toxoplasmosis. mBio. 9(2): e00301-18. *Senior and corresponding author.
- Gaji RY, Johnson D, Wang M, Treeck M, Hudmon A and Arrizabalaga G. (2015). Phosphorylation of a myosin motor by TgCDPK3 facilitates rapid initiation of motility during Toxoplasma gondii egress. PLoS Pathogens. 11(11): e1005268.
- Treeck M, Sanders JL, Gaji RY, Lafavers KA, Child MA, Arrizabalaga G, Elias JE and Boothroyd JC. (2014). CDPK3 of Toxoplasma influences basal calcium levels and functions beyond egress as revealed by quantitative phosphoproteome analysis. PLoS Pathogens, 10(6): e1004197.
- Gaji RY, Checkley L, Reese ML, Ferdig MT and Arrizabalaga G. (2014). Expression of the essential kinase PfCDPK1 from Plasmodium falciparum in Toxoplasma gondii facilitates the discovery of novel antimalarial drugs. Antimicrobial Agents and Chemotherapy, 58 (2598-2607).
- Gaji RY, Huynh M and Carruthers VB. (2013) A Novel High Throughput Invasion Screen Identifies Host Actin Regulators Required for Efficient Cell Entry by Toxoplasma gondii. PLoS One, 8(5):e64693.
- Sloves PJ, Delhaye S, Mouveaux T, Werkmeister E, Gaji RY, Schaeffer-Reiss C, Dorsselear AV, Carruthers VB and Tomavo S. (2012). A crucial role for Toxoplasma SLR in the biogenesis of apical secretory organelles and host infection. Cell Host and Microbe, 11, (515-527).
- Gaji RY, Flammer HP and Carruthers VB. (2011). Forward targeting of Toxoplasma gondii proproteins to the micronemes involves conserved aliphatic amino acids. Traffic, 12 (840-853).
- Gaji RY, Behnke MS, White MW and Carruthers VB. (2011). Cell Cycle-dependent intercellular transmission of Toxoplasma gondii is accompanied by marked changes in parasite gene expression. Molecular Microbiology, 79 (192-204).
- Gaji RY and Howe DK. (2009). The heptanucleotide motif GAGACGC is a key component of a cis-acting promoter element that is critical for SnSAG1 expression in Sarcocystis neurona. Molecular and Biochemical Parasitology, 166 (85-88).
- Howe DK, Gaji RY, Marsh AE, Patil BA, Saville WJ, Lindsay DS, Dubey JP and Granstrom DE. (2007). Strains of sarcocystis neurona exihibit variation in their surface antigens, including the absence of the major surface antigen SnSAG1. International Journal for Parasitology, 38 (623-631).
- Gaji RY, Zhang D, Vaishnava S, Striepen B. and Howe DK. (2006). Molecular genetic transfection of the coocidian parasite Sarcocystis neurona. Molecular and Biochemical Parasitology, 150 (1-9).
- Zhang D, Gaji, RY and Howe DK. (2006). Identification and characterization of a nucleotide triphosphate hydrolase in Sarcocystis neurona. International Journal for Parasitology, 36 (1197-1204).
- Vaishnava S, Morrison, DP, Gaji RY, Murray, JM, Entzeroth, R, Howe DK and Striepen B. (2005). Plastid segregation and cell division in the apicomplexan parasite Sarcocystis neurona. Journal of Cell Science, 118 (3397-407).
- Howe DK, Gaji, RY, Mroz-Brarrett M, Gubbels MJ, Striepen B and Stamper S. (2005). Sarcocystis neurona merozoites express a family of immunogenic surface antigens that are orthologues of the Toxoplasma gondii surface antigens (SAGs) and SAG-related sequences. Infection and Immunity, 73 (1023- 33).
-
Article Item
 Virginia Tech veterinary college gets funding for research into parasite found in cats , article
Virginia Tech veterinary college gets funding for research into parasite found in cats , articleFound in cats, Toxoplasma gondii is a human pathogen with serious health ramifications, causing life-threatening illnesses for people with immunodeficienies, miscarriages in pregnant women, and blindness in newborn children.
Currently we are looking for students interested in pursuing PhD program. If you would like to join our group, send your application to Dr. Gaji at rajgaji@vt.edu.
In the application, include a cover letter describing your education and research experience, copies of your transcripts, TOEFL or IELTS scores. Your application should be a single pdf file.
Students with a masters degree in parasitology or biotechnology would be given preference.
Virginia Tech Center for One Health Research
1410 Prices Fork Road
Blacksburg, VA 24060






