Seleem Laboratory

Welcome to Seleem Lab
Antimicrobial Drug Discovery
Antimicrobial resistance knows no geographic or human-animal boundaries, demanding a One Health-driven approach to combat this escalating threat.
The Seleem lab's overarching objective is to develop novel antimicrobials and enhance drug delivery mechanisms for effectively treating infectious diseases. Additionally, we are dedicated to pioneering innovative techniques for early detection and identification of individual bacteria and fungi within intricate environments.

Drug Repurposing for Antimicrobial Resistance
Drug repurposing is gaining significant momentum due to its advantages, such as bypassing extensive preclinical testing, expediting the progression to clinical efficacy trials, and reducing drug development costs by at least 40%.
At the forefront of this field, the Seleem Laboratory has undertaken groundbreaking research on the mechanism of action of various FDA-approved drugs and clinical molecules to target multidrug-resistant pathogens.
As the research evolved, the lab embarked on more intricate studies, becoming the pioneer in identifying the antimicrobial mechanism of action of several FDA-approved drugs and repurposing them for potential clinical trials.
Currently, our focus lies in utilizing different animal models in conjunction with molecular techniques to assess the potential of repurposed drugs in treating bacterial and fungal infections. The successful continuation of these projects owes much to the support received from National Institutes of Health.

Antimicrobial Drug Discovery
In the realm of de novo drug discovery, Seleem lab has been an active collaborator with researchers and esteemed colleagues from various national and international institutions, in the pursuit of antibacterial and antifungal drug discovery.
Currently, Seleem lab is engaged in multiple collaborative de novo drug discovery projects with researchers both nationally and internationally, furthering the collective effort to combat infectious diseases.
Neisseria gonorrhoeae

Our research team maintains an active drug discovery program with a specific emphasis on identifying compounds that show activity against Neisseria gonorrhoeae.
In collaboration with several partners, we are deeply involved in researching, developing, characterizing, and testing innovative therapeutic approaches to combat drug-resistant pathogens. By working together with our collaborators, we aim to contribute significantly to the advancement of effective treatments against challenging infectious diseases.
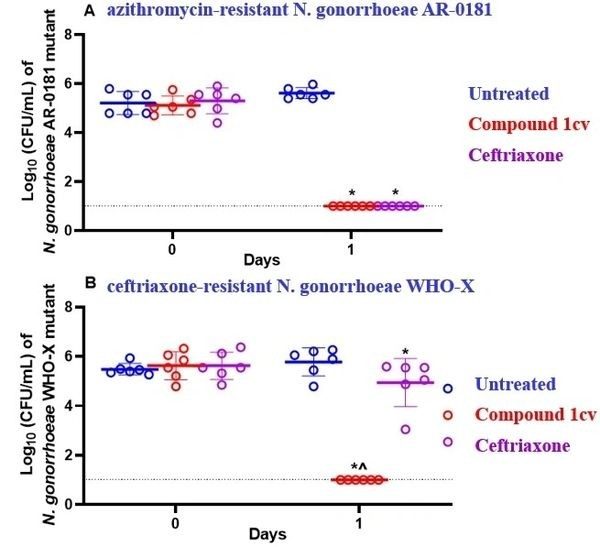
In our gonococcal infection mouse model, we assessed the in vivo efficacy of a single oral dose of compound 1cv against two strains: A) azithromycin-resistant N. gonorrhoeae AR-0181, and B) ceftriaxone-resistant N. gonorrhoeae WHO-X
The data were analyzed via a two-way ANOVA followed by post-hoc Dunnett’s test for multiple comparisons. Asterisks (*) indicate a statistically significant difference for treatment groups compared to the vehicle, while the symbol (^) indicates a statistically significant difference for compound 1cv as compared to a single intraperitoneal dose of 15 mg/kg ceftriaxone (P<0.05).
Clostridium difficile
We have an active drug discovery program with a focus on Clostridium difficile.
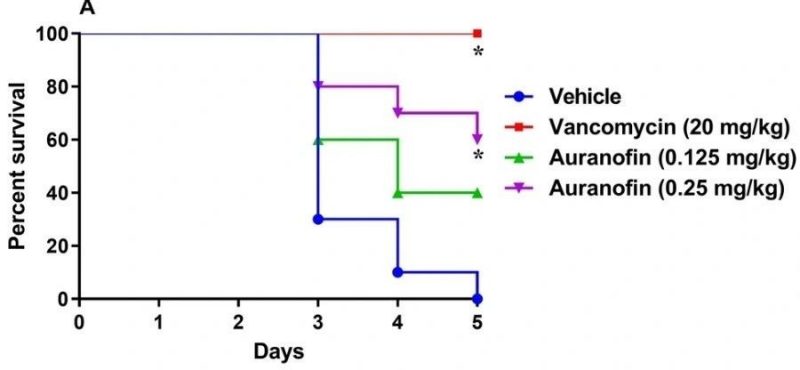
Efficacy of auranofin in treatment of Clostridioides difficile infection in hamsters. Hamsters were treated with auranofin, vancomycin (20 mg/kg), or the vehicle for 5 days after infection with C. difficile UNT103-1. Kaplan–Meier survival curves were analyzed using a log-rank (Mantel–Cox) and Gehan–Breslow–Wilcoxon tests (P < 0.05). An asterisk (*) denotes a statistically significant difference between hamsters treated with either auranofin or vancomycin in comparison with the vehicle-treated hamsters.
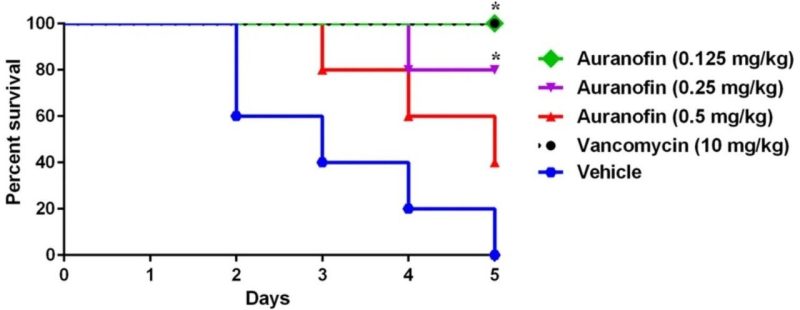
Auranofin protects mice against Clostridioides difficile infection. Mice were treated with auranofin (0.125 mg/kg, 0.25 mg/kg, and 0.5 mg/kg), vancomycin (10 mg/kg), or the vehicle for 5 days after infection with C. difficile spores. Kaplan–Meier survival curves were analyzed using a log-rank (Mantel–Cox) test. Asterisks (*) denote statistical significant difference between mice treated with either auranofin, or vancomycin in comparison with the vehicle-treated mice.
VRE

Burden of VRE (E. faecium HM-952) in the fecal contents of colonized mice. The CFU data were analyzed via a two-way ANOVA with post hoc Dunnett’s test for multiple comparisons. An asterisk (*) indicates a significant difference (P < 0.05) between mice treated with AZM or LZD compared with vehicle. A pound sign (#) indicates a significant difference (P < 0.05) between mice treated with AZM compared to LZD-treated mice.
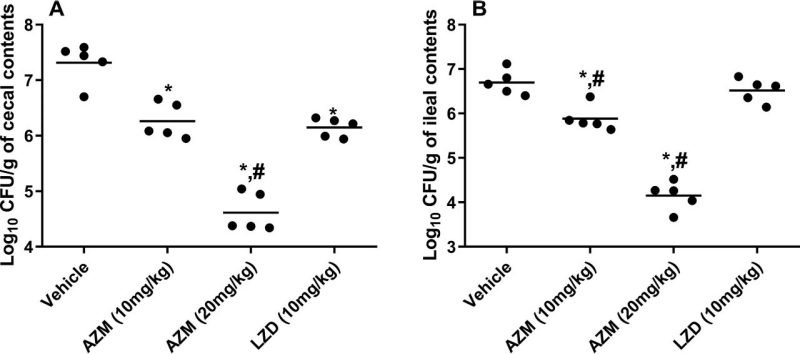
Burden of VRE (E. faecium HM-952) in (A) the cecal contents of colonized mice and (B) the ileal contents of colonized mice (collected at sacrifice on day 8). The CFU data were analyzed via a one-way ANOVA with post hoc Dunnett’s test for multiple comparisons. An asterisk (*) indicates a significant difference (P < 0.05) between mice treated with AZM or LZD compared with untreated mice (vehicle). A pound sign (#) indicates a significant difference (P < 0.05) between mice treated with AZM compared to LZD-treated mice.
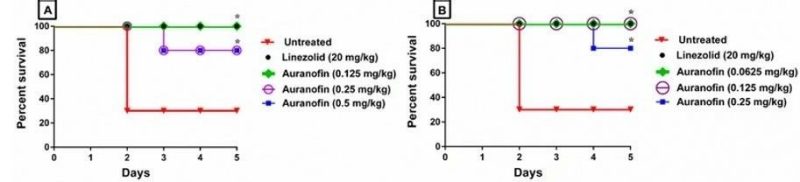
In vivo antibacterial activity of auranofin against E. faecium NR-31909 in the murine septicemia model when administered (A) Orally at 0.125 mg/kg, 0.25 mg/kg and 0.5 mg/kg; and (B) Subcutaneously (S.C.) at 0.0625 mg/kg, 0.125 mg/kg and 0.25 mg/kg compared to the vehicle control and the standard antibiotic linezolid given orally at 20 mg/kg. Mice survival was monitored for 5 days. Results were analyzed for statistical difference utilizing graphpad prism. (*) Denotes significant difference between each treated group and the untreated group (P < 0.05).

MRSA
Mouse model of MRSA systemic infection: Ten mice per group were infected (i.p) with lethal dose of MRSA USA300 and treated orally with auranofin (0.125 or 0.25 mg/kg), linezolid (25 mg/kg), or the vehicle alone for three days (one dose per day). Mice were monitored for five days and the percent survival was calculated. A log rank test was performed using 95% confidence intervals and the statistical significance was calculated in order to compare treated to control groups. P values of (* ≤ 0.05) (**P ≤ 0.01) are considered as significant. Detailed “P” values are listed below. Control vs linezolid (25 mg/kg):0.0001, Control vs auranofin (0.25 mg/kg): 0.0008, Control vs auranofin (0.125 mg/kg): 0.04. (b) Five mice per group were infected (i.p) with non-lethal dose of MRSA USA300 and treated orally with auranofin (0.25 mg/kg), linezolid (25 mg/kg), or the vehicle alone for two days (one dose per day). 24 hours after the last treatment, mice were euthanized and their spleen and liver were excised and homogenized in TSB to count viable MRSA colonies. The number of CFU from each mouse is plotted as individual points. Statistical analysis was conducted using the two-tailed Student’s ‘t’ test and P values of (* ≤ 0.05) are considered as significant. Detailed “P” values are listed below. Spleen: Control vs linezolid (25 mg/kg):0.0173, Control vs auranofin (0.25 mg/kg): 0.0153. Liver: Control vs linezolid (25 mg/kg):0.0481, Control vs auranofin (0.25 mg/kg): 0.0178.
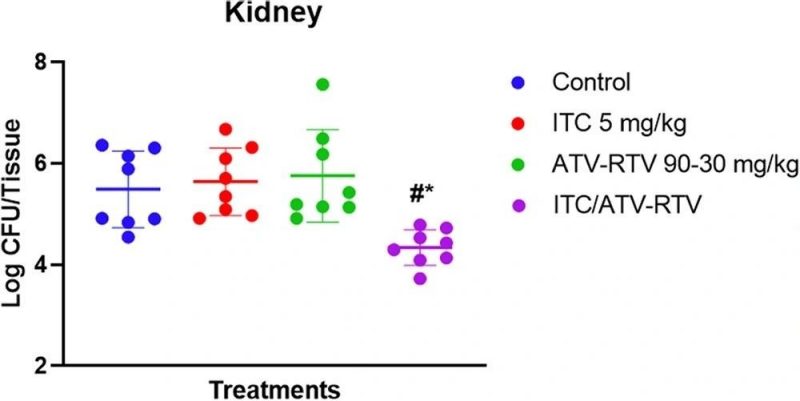
Candida auris mouse model of disseminated candidiasis
In vivo efficacy of the itraconazole (ITC)-atazanavir (ATV)-ritonavir (RTV) combination in a murine model of C. auris disseminated infection. Female CD-1 mice (n = 8) were infected with azole-resistant C. auris AR0390 and then treated with either the vehicle control, ITC (5 mg/kg), ATV-RTV (90 and 30 mg/kg, respectively), or ITC-ATV-RTV (5, 90, and 30 mg/kg, respectively). Statistical difference was measured via one-way analysis of variance (ANOVA) with the post hoc Dunnett’s test for multiple comparisons. The asterisk (*) denotes statistical significance of the combination treatment (P < 0.01) compared to the untreated control. The pound sign (#) denotes statistical significance (P < 0.01) compared to the ITC-treated group.

Changing the Culture of Bacterial Culture
Every year, more than one million people in the United States are affected by bloodstream infections (BSI) and about 270,000 die as a result. Blood specimens are obtained and cultured for 1 to 5 days to determine the identity of existing pathogens and susceptibility to various antimicrobial agents. In an attempt to treat the infection before results of the culture come back, doctors often give patients antibiotics cocktail, hoping that one of the medications in the bunch will cure the patient. Often, it doesn’t, and sometimes patients are harmed by taking drugs they didn’t need. This practice also contributes to the increasing prevalence of antimicrobial resistance. For every hour of delay in starting correct antimicrobial therapy, the risk of death for a given patient with sepsis increases by 6% to 10%. Dr. Seleem has collaborated with Dr. Ji-Xin Cheng, Moustakas Chair Professor in Photonics and Optoelectronics at Boston University and world-wide leader in coherent Raman scattering microscopy, to focus on bloodstream infections and drug resistance. Drs. Seleem and Cheng with a $1.7 million grant from the National Institutes of Health are developing a microsecond-scale stimulated Raman spectroscopic imaging platform to enable in situ identification of a single bacterium/fungus in a complex environment at sub-micron resolution; along with early detection of its response to an antimicrobial drug(13, 14). This development will shift the paradigm of BSI diagnosis from a time-consuming, cultivation-dependent procedure to a culture-independent, in situ approach. Further clinical translation of the proposed technology would save patients’ lives with the early diagnosis of BSI and accurate profiling of a pathogen’s susceptibility to antimicrobials.
- Thangamani, S.; Mohammad, H.; Abushahba, M. F.; Hamed, M. I.; Sobreira, T. J.; Hedrick, V. E.; Paul, L. N.; Seleem, M. N. Exploring simvastatin, an antihyperlipidemic drug, as a potential topical antibacterial agent. Sci Rep 2015, 5, 16407.
- Thangamani, S.; Mohammad, H.; Abushahba, M. F.; Sobreira, T. J.; Seleem, M. N. Repurposing auranofin for the treatment of cutaneous staphylococcal infections. Int J Antimicrob Agents 2016, 47, 195-201.
- Thangamani, S.; Mohammad, H.; Abushahba, M. F.; Sobreira, T. J.; Hedrick, V. E.; Paul, L. N.; Seleem, M. N. Antibacterial activity and mechanism of action of auranofin against multi-drug resistant bacterial pathogens. Sci Rep 2016, 6, 22571.
- Younis, W.; Thangamani, S.; Seleem, M. N. Repurposing Non-Antimicrobial Drugs and Clinical Molecules to Treat Bacterial Infections. Curr Pharm Des 2015, 21, 4106-11.
- Thangamani, S.; Younis, W.; Seleem, M. N. Repurposing celecoxib as a topical antimicrobial agent. Front Microbiol 2015, 6, 750.
- Thangamani, S.; Younis, W.; Seleem, M. N. Repurposing Clinical Molecule Ebselen to Combat Drug Resistant Pathogens. PLoS One 2015, 10, e0133877.
- Thangamani, S.; Younis, W.; Seleem, M. N. Repurposing ebselen for treatment of multidrug-resistant staphylococcal infections. Sci Rep 2015, 5, 11596.
- Thangamani, S.; Mohammad, H.; Younis, W.; Seleem, M. N. Drug repurposing for the treatment of staphylococcal infections. Curr Pharm Des 2015, 21, 2089-100.
- Brezden, A.; Mohamed, M. F.; Nepal, M.; Harwood, J. S.; Kuriakose, J.; Seleem, M. N.; Chmielewski, J. Dual Targeting of Intracellular Pathogenic Bacteria with a Cleavable Conjugate of Kanamycin and an Antibacterial Cell-Penetrating Peptide. J Am Chem Soc 2016, 138, 10945-9.
- Kuriakose, J.; Hernandez-Gordillo, V.; Nepal, M.; Brezden, A.; Pozzi, V.; Seleem, M. N.; Chmielewski, J. Targeting intracellular pathogenic bacteria with unnatural proline-rich peptides: coupling antibacterial activity with macrophage penetration. Angew Chem Int Ed Engl 2013, 52, 9664-7.
- Nepal, M.; Mohamed, M. F.; Blade, R.; Eldesouky, H. E.; T, N. A.; Seleem, M. N.; Chmielewski, J. A Library Approach to Cationic Amphiphilic Polyproline Helices that Target Intracellular Pathogenic Bacteria. ACS Infect Dis 2018, 4, 1300-1305.
- Pei, Y.; Mohamed, M. F.; Seleem, M. N.; Yeo, Y. Particle engineering for intracellular delivery of vancomycin to methicillin-resistant Staphylococcus aureus (MRSA)-infected macrophages. J Control Release 2017, 267, 133-143.
- Hong, W.; Karanja, C. W.; Abutaleb, N. S.; Younis, W.; Zhang, X.; Seleem, M. N.; Cheng, J. X. Antibiotic Susceptibility Determination within One Cell Cycle at Single-Bacterium Level by Stimulated Raman Metabolic Imaging. Anal Chem 2018, 90, 3737-3743.
- Karanja, C. W.; Hong, W.; Younis, W.; Eldesouky, H. E.; Seleem, M. N.; Cheng, J. X. Stimulated Raman Imaging Reveals Aberrant Lipogenesis as a Metabolic Marker for Azole-Resistant Candida albicans. Anal Chem 2017, 89, 9822-9829.
- Ghosh, C.; Yadav, V.; Younis, W.; Mohammad, H.; Hegazy, Y. A.; Seleem, M. N.; Sanyal, K.; Haldar, J. Aryl-alkyl-lysines: Membrane-Active Fungicides That Act against Biofilms of Candida albicans. ACS Infect Dis 2017, 3, 293-301.
- Mohammad, H.; Elghazawy, N. H.; Eldesouky, H. E.; Hegazy, Y. A.; Younis, W.; Avrimova, L.; Hazbun, T.; Arafa, R. K.; Seleem, M. N. Discovery of a Novel Dibromoquinoline Compound Exhibiting Potent Antifungal and Antivirulence Activity That Targets Metal Ion Homeostasis. ACS Infect Dis 2018, 4, 403-414.
- Eldesouky, H. E.; Mayhoub, A.; Hazbun, T. R.; Seleem, M. N. Reversal of Azole Resistance in Candida albicans by Sulfa Antibacterial Drugs. Antimicrob Agents Chemother 2018, 62.
- Eldesouky, H. E.; Li, X.; Abutaleb, N. S.; Mohammad, H.; Seleem, M. N. Synergistic interactions of sulfamethoxazole and azole antifungal drugs against emerging multidrug-resistant Candida auris. Int J Antimicrob Agents 2018.
- Mohammad, H.; Mayhoub, A. S.; Ghafoor, A.; Soofi, M.; Alajlouni, R. A.; Cushman, M.; Seleem, M. N. Discovery and characterization of potent thiazoles versus methicillin- and vancomycin-resistant Staphylococcus aureus. J Med Chem 2014, 57, 1609-15.
- Hagras, M.; Abutaleb, N. S.; Ali, A. O.; Abdel-Aleem, J. A.; Elsebaei, M. M.; Seleem, M. N.; Mayhoub, A. S. Naphthylthiazoles: Targeting Multidrug-Resistant and Intracellular Staphylococcus aureus with Biofilm Disruption Activity. ACS Infect Dis 2018.
- Kotb, A.; Abutaleb, N. S.; Seleem, M. A.; Hagras, M.; Mohammad, H.; Bayoumi, A.; Ghiaty, A.; Seleem, M. N.; Mayhoub, A. S. Phenylthiazoles with tert-Butyl side chain: Metabolically stable with anti-biofilm activity. Eur J Med Chem 2018, 151, 110-120.
- Elsebaei, M. M.; Mohammad, H.; Abouf, M.; Abutaleb, N. S.; Hegazy, Y. A.; Ghiaty, A.; Chen, L.; Zhang, J.; Malwal, S. R.; Oldfield, E.; Seleem, M. N.; Mayhoub, A. S. Alkynyl-containing phenylthiazoles: Systemically active antibacterial agents effective against methicillin-resistant Staphylococcus aureus (MRSA). Eur J Med Chem 2018, 148, 195-209.
- Hagras, M.; Hegazy, Y. A.; Elkabbany, A. H.; Mohammad, H.; Ghiaty, A.; Abdelghany, T. M.; Seleem, M. N.; Mayhoub, A. S. Biphenylthiazole antibiotics with an oxadiazole linker: An approach to improve physicochemical properties and oral bioavailability. Eur J Med Chem 2018, 143, 1448-1456.
- Eid, I.; Elsebaei, M. M.; Mohammad, H.; Hagras, M.; Peters, C. E.; Hegazy, Y. A.; Cooper, B.; Pogliano, J.; Pogliano, K.; Abulkhair, H. S.; Seleem, M. N.; Mayhoub, A. S. Arylthiazole antibiotics targeting intracellular methicillin-resistant Staphylococcus aureus (MRSA) that interfere with bacterial cell wall synthesis. Eur J Med Chem 2017, 139, 665-673.
- Mohammad, H.; Younis, W.; Ezzat, H. G.; Peters, C. E.; AbdelKhalek, A.; Cooper, B.; Pogliano, K.; Pogliano, J.; Mayhoub, A. S.; Seleem, M. N. Bacteriological profiling of diphenylureas as a novel class of antibiotics against methicillin-resistant Staphylococcus aureus. PLoS One 2017, 12, e0182821.
- Hagras, M.; Mohammad, H.; Mandour, M. S.; Hegazy, Y. A.; Ghiaty, A.; Seleem, M. N.; Mayhoub, A. S. Investigating the Antibacterial Activity of Biphenylthiazoles against Methicillin- and Vancomycin-Resistant Staphylococcus aureus (MRSA and VRSA). J Med Chem 2017, 60, 4074-4085.
- Eissa, I. H.; Mohammad, H.; Qassem, O. A.; Younis, W.; Abdelghany, T. M.; Elshafeey, A.; Abd Rabo Moustafa, M. M.; Seleem, M. N.; Mayhoub, A. S. Diphenylurea derivatives for combating methicillin- and vancomycin-resistant Staphylococcus aureus. Eur J Med Chem 2017, 130, 73-85.
- Yahia, E.; Mohammad, H.; Abdelghany, T. M.; Fayed, E.; Seleem, M. N.; Mayhoub, A. S. Phenylthiazole antibiotics: A metabolism-guided approach to overcome short duration of action. Eur J Med Chem 2017, 126, 604-613.
- Opoku-Temeng, C.; Naclerio, G. A.; Mohammad, H.; Dayal, N.; Abutaleb, N. S.; Seleem, M. N.; Sintim, H. O. N-(1,3,4-oxadiazol-2-yl)benzamide analogs, bacteriostatic agents against methicillin- and vancomycin-resistant bacteria. Eur J Med Chem 2018, 155, 797-805.
- Yin, X.; Mohammad, H.; Eldesouky, H. E.; Abdelkhalek, A.; Seleem, M. N.; Dai, M. Rapid synthesis of bicyclic lactones via palladium-catalyzed aminocarbonylative lactonizations. Chem Commun (Camb) 2017, 53, 7238-7241.
- Lv, W.; Banerjee, B.; Molland, K. L.; Seleem, M. N.; Ghafoor, A.; Hamed, M. I.; Wan, B.; Franzblau, S. G.; Mesecar, A. D.; Cushman, M. Synthesis of 3-(3-aryl-pyrrolidin-1-yl)-5-aryl-1,2,4-triazines that have antibacterial activity and also inhibit inorganic pyrophosphatase. Bioorg Med Chem 2014, 22, 406-18.
- AbdelKhalek, A.; Abutaleb, N. S.; Mohammad, H.; Seleem, M. N. Antibacterial and antivirulence activities of auranofin against Clostridium difficile. Int J Antimicrob Agents 2018.
Current Lab Members

Mohamed Seleem, DVM, PhD
Tyler & Frances Young Endowed Chair Bacteriology
Director- Center for One Health Research (COHR), Virginia Tech
seleem@vt.edu
Professor Christopher Lawrence, PhD
Group Manager
Center for One Health Research (COHR)
cblawren@vt.edu
Mohamed F. Mohamed, DVM, PhD
Research Scientist
cblawren@vt.edu

Nader Shawky Abutaleb, PharmD, PhD
Postdoc
nabutale@purdue.edu

Ehab Ali Salama, PharmD, PhD
Postdoc
ehabsalama@vt.edu
Autumn Dove, PhD
Postdoc
autumndove@vt.edu
Ahmed Abouelkhair, DVM, MVSc
4th Year, Ph.D. candidate
aabouelkhair@vt.edu

Nour Alkashef, PharmD, MS
4th Year, Ph.D. candidate
nouralkashef@vt.edu

Abdallah (Adam) Abdelsattar, MS
3rd Year, Ph.D. candidate
abdelsattar@vt.edu

Brice Stolz
3rd Year, Ph.D. candidate
bricestolz@vt.edu

Ammar Khan, PharmD, MS
2nd Year, Ph.D. Student
ammarakhan@vt.edu

Somaia Abdelmegeed
2nd Year, Master's Student
somaiamahmoud@vt.edu
Alumni
- Haroon T Mohammad, Postdoc (2017-2020)
- Yehia El-Gammal, PhD (2020-2024)
- Rusha Pal, PhD (2018-2022)
Postdoc at Harvard Medical School
- Hassan Eldesouky, PharmD, PhD (2016-2020)
Postdoc Washington State University
- Ahmed Abdelkhalek Hassan, PharmD, PhD (2015-2020)
Assistant Professor, Purdue University
Google Scholar Profile - Haroon T Mohammad, PhD (2011-2017)
- Shankar Thangamani, DVM, PhD (2013-2016)
Assistant Professor, Purdue University
The Thangamani Lab
Google Scholar Profile - Mohamed F. Mohamed, DVM, PhD (2012-2016)
Rush University Medical Center
- Babatomiwa Kikiowo, M.S. (2022-2024)
- Nicolas Burns, M.S. (2022-2023)
- Hsin-Wen Liang, M.S. (2020-2023)
Ph.D. student at Kim Lewis lab, Northeastern University
- Omar Gehad Sadiek, Purdue University (Fall 2019- Spring 2020)
- Guanming Jiao
Ph.D. student, Purdue University
Trained Fall 2019-Spring 2020 - Shukun Wang
Ph.D. student, Purdue University
Trained Fall 2019-Spring 2020 - Bo Huang
Ph.D. student, Purdue University
Trained Fall 2019-Spring 2020 - Amr Kais
Indiana University School of Medicine
Trained Summer 2019-Spring 2020 - Marwa Alhashimi
Ph.D. student, Purdue University
Trained Fall 2018-Spring 2020 - Ahmed Elkashif
Ph.D. student, Purdue University
Trained Spring 2018-Spring 2020 - Khadija Elmagarmid
High School Student
Trained Summer 2019 - Daoyi Li
Ph.D. Student, Purdue University
Trained August 2018- July 2019 - Deepansh Mody
M.S. Student, Purdue University
Trained January 2018- May 2019 - Alsagher Ali, DVM, Ph.D.
Professor, South Valley University
Visiting Scholar 2018 - Young Jin Seong
Ph.D. student, Purdue University
Trained 2018 - Jelan AbdelRazik, PharmD, Ph.D.
Associate Professor, Department of Industrial Pharmacy , Faculty of Pharmacy, ِِAssiut University
Visiting Scholar 2018 - Xiaoyan Li, Ph.D.
Lecturer Department of Life Sciences at Northeast Forestry University in Harbin, China
Visiting Scholar 2017-2018 - Khalifa Elmagarmid
Research Assistant, Children's Hospital Los Angeles (CHLA)
Trained Summer 2017 and 2018 - Youssef Ahmad Hegazy, PharmD
Ph.D. student, Purdue University
Trained 2017 - Mostafa Abushahba, DVM, Ph.D.
School of Medicine, Washington University, St Louis
Visiting Scholar 2014-2016 - Maha Hamed, DVM, Ph.D.
Professor of Infectious Diseases, Faculty of Veterinary Medicine, Assiut University
Visiting Scholar 2014 and 2016 - Omar Kamel Amen, DVM, Ph.D.
Department Head Poultry Diseases, Assiut University
Visiting Scholar 2015 - Waleed Younis, DVM, Ph.D.
Assistant Professor, South Valley University,
Visiting Scholar 2013-2015 - Ruba Alajlouni, MSc
Scientist at Solid State Chemical Information, USA
Trained 2011-2013 - Adil Ghafoor, MD
Indiana University School of Medicine
Trained 2011-2012 - Muhammad Soofi, MD
The Ohio State University College of Medicine
Trained 2011-2012
- Mohammed Ali, DVM, Ph.D.
Assistant Professor, Dept. of Forensic Med and Vet Toxicology, Assiut University - Ahmad Athamneh, Ph.D.
Kindi Therapeutics and Drug Discovery LLC
Research Associate 2017-2018 - Dr. Usama Mahmoud, DVM, Ph.D.
Associate Professor, College of Veterinary Medicine, Assiut University, Egypt
- Ahmed Elhassanny, PharmD, Ph.D.
Postdoc
- Abey Bandara, Ph.D.
Research Associate Professor

Mohamed Seleem, DVM, MS, PhD
Tyler J. and Frances F. Young Chair in Bacteriology
Professor
Department of Biomedical Sciences and Pathobiology
VA-MD College of Veterinary Medicine
Virginia Tech
205 Duck Pond Drive
Blacksburg, VA 24061
seleem@vt.edu
PhD, Microbiology and Biotechnology, 2006
Virginia-Maryland College of Veterinary Medicine
Virginia Tech
Blacksburg, VA
MVSc, Zoonoses, 2000
College of Veterinary Medicine
Assiut University
Assiut, Egypt
BVSc and AS, 1995
College of Veterinary Medicine
Assiut University
Assiut, Egypt
The long-term goal of the Seleem research program is focused on developing new antimicrobials and improving delivery of drugs for the treatment of infectious diseases. The group is also developing novel methods for early detection and identification of a single bacterium/fungus in a complex environment.
2020–present
Tyler J. and Frances F. Young Chair in Bacteriology
Department of Biomedical Sciences and Pathobiology
Virginia-Maryland College of Veterinary Medicine
Virginia Tech
Blacksburg, VA
2018–2020
Professor
Department of Comparative Pathobiology
College of Veterinary Medicine
Purdue University
West Lafayette, IN
2015–2018
Associate Professor
Department of Comparative Pathobiology
College of Veterinary Medicine
Purdue University
West Lafayette, IN
2011–2015
Assistant Professor
Department of Comparative Pathobiology
College of Veterinary Medicine
Purdue University
West Lafayette, IN
2007–2009
Postdoctoral Research
Associate Institute for Critical Technology and Applied Science (ICTAS)
Virginia Tech
Blacksburg, VA
2006–2007
Postdoctoral Research Associate
College of Veterinary Medicine
Cornell University
Ithaca, NY
2001–2006
Research and Teaching Assistant
Virginia-Maryland College of Veterinary Medicine
Virginia Tech
Blacksburg, VA
1996–2001
Assistant Lecturer and Research Scientist
College of Veterinary Medicine
Assiut University
Assiut, Egypt
- Seed for Success Award, Purdue University, 2019
The Seed for Success Award is given in recognition of the accomplishments of investigators for their efforts in obtaining a $1 million dollar or more external sponsored award. - PVM Excellence in Research Award, Purdue University, 2019
The award honors faculty at the College of Veterinary Medicine who have demonstrated dedication and excellence in research. - Seed for Success Award, Purdue University, 2017
The Seed for Success Award is given in recognition of the accomplishments of investigators for their efforts in obtaining a $1 million dollar or more external sponsored award. - Purdue University Faculty Scholar, 2016
The University Faculty Scholars Program recognizes outstanding faculty members who are on an accelerated path for academic distinction. - Zoetis Excellence in Research Award, Purdue University, 2015
The award recognizes outstanding research effort and productivity. - Zoetis Distinguished Veterinary Teacher Award, Purdue University, 2014
The award recognizes educators for their character and leadership qualities, as well as their outstanding teaching abilities. - Outstanding Dissertation Award in Sciences and Engineering, Virginia Tech, 2007
The award recognizes the outstanding PhD thesis in sciences and engineering earned in 2006.
213. M. S. Youse, N. S. Abutaleb, A. Nocentini, A. S. Abdelsattar, F. Ali, C. T. Supuran, M. N. Seleem, D. P. Flaherty. Optimization of Ethoxzolamide Analogs with Improved Pharmacokinetic Properties for In Vivo Efficacy against Neisseria gonorrhea. Journal of Medicinal Chemistry, 2024. https://doi.org/10.1021/acs.jmedchem.4c01187
212. Alkashef, N.M. and Seleem, M.N., 2024. Novel combinatorial approach: Harnessing HIV protease inhibitors to enhance amphotericin B’s antifungal efficacy in cryptococcosis. PloS one, 19(8), p.e0308216. https://doi.org/10.1371/journal.pone.0308216
211. A. Abouelkhair and M. N. Seleem. (2024). Exploring Novel Microbial Metabolites and Drugs for Inhibiting Clostridioides difficile. mSphere. https://doi.org/10.1128/msphere.00273-24
210. B. Alessandro, N. Alessio, S. Giovannuzzi, N. Paoletti, A. Ammara, S. Bua, N. Abutaleb, A. S. Abdelsattar, C. Capasso, P. Gratteri, D. Flaherty, M. N. Seleem; C. Supuran. (2024). Development of penicillin-based carbonic anhydrase inhibitors targeting multidrug-resistant Neisseria gonorrhoeae: Journal of Medicinal Chemistry, 2024, 67, 11, 9613–9627. https://doi.org/10.1021/acs.jmedchem.4c00740
209. S. Sedaghat, A. Krishnakumar, V. Selvamani, J. Barnard, S. Nejati, H. Wang, D. Detwiler, M. N. Seleem, R. Rahimi. (2024). Laser-Assisted Surface Alloying of Titanium with Silver to Enhance Antibacterial and Bone-Cell Mineralization Properties of Orthopedic Implants. Journal of Materials Chemistry B - 2024 May 8;12(18):4489-4501. DOI: 10.1039/d3tb02481d
208. E. A Salama, Y. Elgammal, A. Wijeratne, N. A Lanman, S. M. Utturkar, A. Farhangian, J. Li, B. Meunier, T. R Hazbun*, M. N. Seleem*. (2024). Lansoprazole interferes with fungal respiration and acts synergistically with amphotericin B against multidrug-resistant Candida auris. Emerging Microbes & Infections. 2322649. https://doi.org/10.1080/22221751.2024.2322649.
207. M. Zhang, H. Ni, X. Ge, L. Lan, M. Seleem, M. Wagner, J. Cheng. (2024). Rapid identification and antimicrobial susceptibility testing in urinary tract infection by FISH-SRS. Advanced Chemical Microscopy for Life Science and Translational Medicine 2024, PC128550F. https://doi.org/10.1117/12.3003454
206. Elgammal, Y., Salama, E.A. and Seleem, M.N., 2024. Enhanced antifungal activity of posaconazole against Candida auris by HIV protease inhibitors, atazanavir, and saquinavir. Scientific Reports, 14(1), p.1571. https://doi.org/10.1038/s41598-024-52012-8
205. Hagras, M., Abuelkhir, A.A., Abutaleb, N.S., Helal, A.M., Fawzy, I.M., Hegazy, M., Seleem, M.N. and Mayhoub, A.S., 2024. Novel phenylthiazoles with a tert-butyl moiety: promising antimicrobial activity against multidrug-resistant pathogens with enhanced ADME properties. RSC advances, 14(2), pp.1513-1526. https://doi.org/10.1039/D3RA07619A
204. Giovannuzzi, S., Marapaka, A. K., Abutaleb, N. S., Carta, F., Liang, H. W., Nocentini, Pisano, L., Seleem, M.N., Flaherty, D.P. and Supuran, C.T. (2023). Inhibition of pathogenic bacterial carbonic anhydrases by monothiocarbamates. Journal of Enzyme Inhibition and Medicinal Chemistry, 38(1), 2284119. https://doi.org/10.1080/14756366.2023.2284119
203. B. Kikiowo, A. B. Bandara, N. S. Abutaleb, M. N. Seleem. (2023). Colonization efficiency of multidrug-resistant Neisseria gonorrhoeae in a female mouse model. Pathogens and Disease, ftad030, https://doi.org/10.1093/femspd/ftad030 (October 2023).
202. Pal, R., & Seleem, M. N. (2023). Antisense inhibition of RNA polymerase α subunit of Clostridioides difficile. Microbiology Spectrum, e01755-23. https://doi.org/10.1128/spectrum.01755-23
201. M. Hagras, N. S. Abutaleb, H. G. Ezzat, E. A. Salama, M. N. Seleem and Abdelrahman S. Mayhoub. (2023). Naphthylthiazoles:Broad Spectrum Class of Antifungals. RSC Med. Chem., 2023. https://doi.org/10.1039/D3MD00323J
200. H. Almolhim, A. Elhassanny, N. Abutaleb, A. S. Abdelsattar, M. N. Seleem, P. Carlier. (2023). Substituted salicylic acid analogs offer improved potency against multidrug-resistant Neisseria gonorrhoeae and good selectivity against commensal vaginal bacteria. Scientific Reports volume 13, Article number: 14468 (2023). https://doi.org/10.1038/s41598-023-41442-5
199. R. A. Wagdy, N. S. Abutaleb, R. K. Fathalla, Y. Elgammal, S. Weck, R. Pal, P. D. Fischer, C. Ducho, A. Abadi, M. N. Seleem, M. Engel, M. Abdel-Halim. (2023). Discovery of 1,2-diaryl-3-oxopyrazolidin-4-carboxamides as a new class of MurA enzyme inhibitors and characterization of their antibacterial activity. European Journal of Medicinal Chemistry. 2023, 115789. https://doi.org/10.1016/j.ejmech.2023.115789
198. Y. Elgammal, E. Salama, M. N. Seleem. (2023). Saquinavir potentiates itraconazole's antifungal activity against multidrug-resistant Candida auris in vitro and in vivo. Medical Mycology. myad081. https://doi.org/10.1093/mmy/myad081
197. M. Omara, M. Hagras, M. M. Elsebaie, N. S. Abutaleb, H. T. Nour El-Din, M. O. Mekhail, A. S. Attia, M. N. Seleem, M. T. Sarg, A. S. Mayhoub. (2023). Exploring novel aryl/heteroaryl-isosteres of phenylthiazole against multidrug-resistant bacteria. RSC Adv, 2023 Jun 29; 13(29): 19695–19709. https://doi.org/10.1039/D3RA02778C
196. E. A. Salama, H. E. Eldesouky, Y. Elgammal, N. S. Abutaleb, M. N. Seleem. (2023). Lopinavir and ritonavir act synergistically with azoles against Candida auris in vitro and in a mouse model of disseminated candidiasis. International Journal of Antimicrobial Agents, 106906. https://doi.org/10.1016/j.ijantimicag.2023.106906
195. M. Zhang, P.Dong, H. Eldesouky, Y. Zhan, H. Lin, Z. Wang, E. Salama, S. Jusurf, C. Zong, Z.Chen, M. N. Seleem, J. Cheng. (2023). Fingerprint SRS Imaging Unveils Ergosteryl Ester as a Metabolic Signature of Azole-Resistant Candida albicans. Analytical Chemistry. June 13, 2023ac-2023-00900u https://doi.org/10.1021/acs.analchem.3c00900
194. A. Roth, A. Krishnakumar, R. McCain, K. Maruthamuthu, M. McIntosh, Y. Chen, A. Cox, J. Hopf, N. Amber, M. N. Seleem, R. Rahimi. (2023). Biocompatibility and Safety Assessment of Combined Topical Ozone and Antibiotics for Treatment of Infected Wounds. ACS Biomaterials Science & Engineering. https://pubs.acs.org/doi/10.1021/acsbiomaterials.2c01548
193. D. Liu; X. Bai, H. Helmick, M. Samaddar, M. Amalaradjou, X. Li, S. Tenguria, N. Gallina, L. Xu, R. Drolia, U. Aryal, G. Moreira, M. Hust, M.N. Seleem, J. Kokini, R. Ostafe, A. Cox, A. K. Bhunia. (2023). Cell Surface Anchoring of Listeria Adhesion Protein on L. monocytogenes is Fastened by Internalin B for Pathogenesis. Cell Reports. 42(5):112515. https://doi.org/10.1016/j.celrep.2023.112515
192. Elgammal, Y., Salama, E. A., & Seleem, M. N. (2023). Atazanavir Resensitizes Candida auris to Azoles. Antimicrobial Agents and Chemotherapy, e01631-22. https://doi.org/10.1128/aac.01631-22
191. NS Abutaleb, A Shrinidhi, AB Bandara, M. N Seleem, DP Flaherty (2023). Evaluation of 1, 3, 4-Thiadiazole Carbonic Anhydrase Inhibitors for Gut Decolonization of Vancomycin-Resistant Enterococci. ACS Medicinal Chemistry Letters. March 21, 2023. https://doi.org/10.1021/acsmedchemlett.3c00032
190. HTN El-Din, MM Elsebaie, NS Abutaleb, AM Kotb, AS Attia, M. N. Seleem, A. Mayhoub. (2023). Expanding the structure–activity relationships of alkynyl diphenylurea scaffold as promising antibacterial agents. RSC Medicinal Chemistry 14 (2), 367-377. https://doi.org/10.1039/D2MD00351A
189. S. Bose, C. N. Steussy, D. López-Pérez, T. Schmidt, S. C. Kulathunga, M. N. Seleem, M. Lipton, A. D. Mesecar, V. W. Rodwell & C. V. Sauffacher. (2023). Targeting Enterococcus faecalis HMG-CoA reductase with a non-statin inhibitor. Nature Communications biology 6, Article number: 360 (2023). https://doi.org/10.1038/s42003-023-04639-y
188. E. M.E. Dokla, N. S. Abutaleb, S. N. Milik, E. Kindil, O. Assem, Y. Elgammal, M. Nasr, M. McPhillie, K. Abouzid, M N. Seleem, P. Imming, and M. Adel. (2023). SAR investigation and optimization of benzimidazole-based derivatives as antimicrobial agents against Gram-negative bacteria. European Journal of Medicinal Chemistry. Volume 247, 5 February 2023, 115040. https://doi.org/10.1016/j.ejmech.2022.115040
187. A. Marapaka, A. Nocentini, M. S. Youse, W. An, K. J. Holly, C. Das, R. Yadav, M. N. Seleem, C. T. Supuran, and D. P. Flaherty. (2023). Structural Characterization of Thiadiazolesulfonamide Inhibitors Bound to Neisseria gonorrhoeae α-Carbonic Anhydrase. ACS Med. Chem. Lett. 2023, 14, 1, 103-109. https://doi.org/10.1021/acsmedchemlett.2c00471
186. A. M. Sayed, N. S. Abutaleb, A. Kotb, H. G. Ezzat, M. N. Seleem, A. S. Mayhoub, M. M. Elsebaie. (2023). Arylpyrazole as selective anti-enterococci; synthesis and biological evaluation of novel derivatives for their antimicrobial efficacy. J Heterocyclic Chem.2023;60:134–144. https://doi.org/10.1002/jhet.4570
185. Pal R, Seleem MN (2022) Discovery of a novel natural product inhibitor of Clostridioides difficile with potent activity in vitro and in vivo. PLoS ONE 17(8): e0267859. https://doi.org/10.1371/journal.pone.0267859
184. C. W. Karanja, N. Naganna, N. S. Abutaleb, N. Dayal, K. Onyedibe, U. Aryal, M. N. Seleemand H. O. Sintim. (2022). Isoquinoline Antimicrobial Agent: Activity against Intracellular Bacteria and Effect on Global Bacterial Proteome. Molecules 2022, 27(16), 5085; https://doi.org/10.3390/molecules27165085
183. Roth, M. Maruthamuthu, S. Nejati, A. Krishnakumar, V. Selvamani, S. Sedaghat, J. Nguyen, M. N. Seleem& R. Rahimi. (2022). Wearable adjunct ozone and antibiotic therapy system for treatment of Gram-negative dermal bacterial infection. Scientific Reports, 12: 13927 (2022). https://doi.org/10.1038/s41598-022-17495-3
182. W. An, K. J Holly, A. Nocentini, R. Imhoff, C. Hewitt, N. S. Abutaleb, X. Cao, M. N. Seleem, C. T. Supuran, D. P. Flaherty. (2022). Structure-activity relationship studies for inhibitors for vancomycin-resistant Enterococcus and human carbonic anhydrases. Journal of Enzyme Inhibition and Medicinal Chemistry 37 (1), 1838-1844. https://doi.org/10.1080/14756366.2022.2092729
181. M. Elsebaie, H. Nour Din, N. S. Abutaleb, A. Abuelkhir, H. Liang, A. Attia, M N Seleem and A. Mayhoub. (2022). Exploring the Structure-Activity Relationships of Diphenylurea as an Antibacterial Scaffold Active against Methicillin- and Vancomycin-Resistant Staphylococcus aureus. Eur J Med Chem 2022, EJMECH-D-21-03184. https://doi.org/10.1016/j.ejmech.2022.114204
180. R. Pal, A. Athamneh, R. Deshpande, J. Ramirez, K. Adu, P. Muthuirulan, S. Pawar, M. Biazzo, Y. Apidianakis, U. Sundekilde, C. Fuente-Nunez, M. Martens, G. Tegos, M N Seleem. (2022). Probiotics: insights and new opportunities for Clostridioides difficile intervention. Critical Reviews in Microbiology. 2022.2072705. https://doi.org/10.1080/1040841X.2022.2072705
179. H. Oliveira, R. Castelli, L. Alves, J. Nosanchuk, E. Salama, M N. Seleem, M. Rodrigues. (2022). Identification of 4 compounds from the Pharmakon library with antifungal activity against Candida auris and species of Cryptococcus. Medical Mycology. myac033. https://doi.org/10.1093/mmy/myac033
178. G. Naclerio, N. Abutaleb, K. Onyedibe, C. Karanja, H. Eldesouky, H. Liang, A. Dieterly, U. Aryal, T. Lyle, M. N. Seleem, H. O Sintim. 2022. Mechanistic Studies and In Vivo Efficacy of an Oxadiazole-Containing Antibiotic . Journal of Medicinal Chemistry. 2022, 65, 9, 6612–6630. https://doi.org/10.1021/acs.jmedchem.1c02034
177. AEM Elhassanny, NS Abutaleb, MN Seleem. (2022). Auranofin exerts antibacterial activity against Neisseria gonorrhoeae in a female mouse model of genital tract infection. Plos one 17 (4), e0266764. https://doi.org/10.1371/journal.pone.0266764
176. M Zhang, M.N. Seleem, JX Cheng. (2022). Rapid Antimicrobial Susceptibility Testing by Stimulated Raman Scattering Imaging of Deuterium Incorporation in a Single Bacterium. JoVE (Journal of Visualized Experiments), e62398. http://doi.org/10.3791/62398
175. S. Giovannuzzi, N. S. Abutaleb, C. S. Hewitt, F. Carta, A. Nocentini, M N Seleem, D. Flaherty, C. T Supuran. (2022). Dithiocarbamates effectively inhibit the α-carbonic anhydrase from Neisseria gonorrhoeae. Journal of Enzyme Inhibition and Medicinal Chemistry. 2022, VOL. 37, NO. 1, 1-8. https://doi.org/10.1080/14756366.2021.1988945
174. N. S. Abutaleb, A. Elhassanny, A. Nocentini, C. Hewitt, A. Elkashif, B. Cooper, C. T Supuran, M N Seleem, D. Flaherty. (2022). Repurposing FDA-approved sulphonamide carbonic anhydrase inhibitors for treatment of Neisseria gonorrhoeae. Journal of Enzyme Inhibition and Medicinal Chemistry. 2022, VOL. 37, NO. 1, 51-61. https://doi.org/10.1080/14756366.2021.1991336
173. N. S. Abutaleb, A. Elhassanny, M N Seleem. (2022). In vivo efficacy of acetazolamide in a mouse model of Neisseria gonorrhoeae infection. 2022.105454. https://doi.org/10.1016/j.micpath.2022.105454
172. M. Hagras, N. S. Abutaleb, A. M Sayed, E. A. Salama, M N Seleem, A. Mayhoub. (2021). Evaluation of bisphenylthiazoles as a promising class for combating multidrug-resistant fungal infections. Plos one e0258465. https://doi.org/10.1371/journal.pone.0258465
171. Z. Iqbal, H. Hussain, M N Seleem, M. Shabbir, A. Sattar, A.Aqib, X. Kuang, A. Ihsan, H. Hao. (2021). RNA-seq-based transcriptome analysis of a cefquinome-treated, highly resistant, and virulent MRSA strain. Microbial Pathogenesis. 2021 Nov;160:105201. https://doi.org/10.1016/j.micpath.2021.105201
170. H. Mohammad, N. S. Abutaleb, A. M. Dieterly, L. T. Lyle & M. N. Seleem. (2021). Investigating auranofin for the treatment of infected diabetic pressure ulcers in mice and dermal toxicity in pigs. Scientific Reports 11, 10935 (2021). https://doi.org/10.1038/s41598-021-90360-x
169. M. Hamed and M. N. Seleem. (2021). Evaluation of Short Synthetic Antimicrobial Peptides against Staphylococcus pseudintermedius. Journal of Advanced Veterinary Research 11,2,(2021) 69-72. https://advetresearch.com/index.php/AVR/article/view/652/459
168. R. Pal, M. Dai, & M. N. Seleem. (2021). High-throughput screening identifies a novel natural product-inspired scaffold capable of inhibiting Clostridioides difficile in vitro. Scientific Reports 11, 10913 (2021). https://doi.org/10.1038/s41598-021-90314-3
167. H. Hussain, A. Aqib, M.N. Seleem , M. Shabbir, H. Hao, Z. Iqbal, M. Kulyar, T. Zaheer , K. Li. (2021). Genetic basis of molecular mechanisms in β-lactam resistant gram-negative bacteria. Jun10;158:105040. Micro. Patho. 158, 2021, 105040. https://doi.org/10.1016/j.micpath.2021.105040
166. Woodhouse, S. Nejati, V. Selvamani, H. Jiang, S. Chittiboyina, J. Grant, Z. Mutlu, J. Waimin, N. S. Abutaleb, M. N. Seleem, R. Rahimi (2021). Flexible Microneedle Array Patch for Chronic Wound Oxygenation and Biofilm Eradication ACS Appl. Bio Mater. 2021, 4, 7, 5405–5415. https://doi.org/10.1021/acsabm.1c00087
165. S. A Yuk, H. Kim, N. S. Abutaleb, A. M. Dieterly, M. S. Taha, M. D. Tsifansky, L. T. Lyle, M. N. Seleem, Y. Yeo. (2021). Nanocapsules modify membrane interaction of polymyxin B to enable safe systemic therapy of Gram-negative sepsis. Science Advances 7 (32), eabj1577. http://doi.org/10.1126/sciadv.abj1577
164. D P Flaherty, M N Seleem, C T Supuran. (2021). Bacterial carbonic anhydrases: underexploited antibacterial therapeutic targets. Future Medicinal Chemistry fmc-2021-0207. https://doi.org/10.4155/fmc-2021-0207
163. N.S. Abutaleb, A. Elhassanny, D.P. Flaherty, M.N. Seleem. (2021). In vitro and in vivo activities of the carbonic anhydrase inhibitor, dorzolamide, against vancomycin-resistant enterococci. PeerJ 9:e11059. https://doi.org/10.7717/peerj.11059
162. N.S. Abutaleb, and M.N. Seleem. (2021). In vivo efficacy of auranofin in a hamster model of Clostridioides difficile infection. Scientific Reports, 11: 7093 (2021). https://doi.org/10.1038/s41598-021-86595-3
161. C. Hewitt, N.S. Abutaleb, A. Elhassanny, A. Nocentini, X. Cao, D. Amos, M. Youse, K. Holly, A. Marapaka, W. An, J. Kaur, A. Krabill, A. Elkashif, Y. Elgammal, A. L Graboski, C. T. Supuran, M. N. Seleem, D. P. Flaherty. (2021). Structure–Activity Relationship Studies of Acetazolamide-Based Carbonic Anhydrase Inhibitors with Activity against Neisseria gonorrhoeae. ACS Infectious Diseases https://doi.org/10.1021/acsinfecdis.1c00055
160. C. Ghosh, S. Varela‚ÄêAramburu, H. E Eldesouky, S. Hossainy, M. N. Seleem, T. Aebischer, P. H Seeberger. (2021). Non‚ÄêToxic Glycosylated Gold Nanoparticle‚ÄêAmphotericin B Conjugates Reduce Biofilms and Intracellular Burden of Fungi and Parasites. Advanced Therapeutics, 2021,2000293. https://doi.org/10.1002/adtp.202000293
159. M. Zhang, W. Hong, N. S. Abutaleb, J. Li, P. Dong, C. Zong, P. Wang, M. N. Seleem, J. Cheng. (2021). Rapid determination of antimicrobial susceptibility by stimulated Raman scattering imaging of D2O metabolic incorporation in a single bacterium. International Society for Optics and Photonics, 11656; 116560B. https://doi.org/10.1002/advs.202001452
158. E. Coscia, N. Abutaleb, B. Hostette, M. N. Seleem, G. Breur, R. McCain, C. Crain, O. Slaby, M. Capoor, A. McDowell, F. Ahmed, V. Vijayanpillai, S. Narayanan and M. Coscia (2021). Sheep as a Potential Model of Intradiscal Infection by the Bacterium Cutibacterium acnes. Veterinary Sciences 8(48):48. https://doi.org/10.3390/vetsci8030048
157. G. A Naclerio, N. S Abutaleb, M. Alhashimi, M. N. Seleem, H. O Sintim. (2021). N-(1, 3, 4-Oxadiazol-2-yl) Benzamides as Antibacterial Agents against Neisseria gonorrhoeae. International journal of molecular sciences. 22;5;2427. https://doi.org/10.3390/ijms22052427
156. N.S. Abutaleb, A. Elkashif, D.P. Flaherty, M.N. Seleem. (2021). In vivo antibacterial activity of acetazolamide. Antimicrobial Agents and Chemotherapy 10.1128/AAC.01715-20. https://doi.org/10.1128/AAC.01715-20
155. H. E. Eldesouky, E. A. Salama, N. A. Lanman, T. R. Hazbun, M.N. Seleem. (2021). Potent Synergistic Interactions between Lopinavir and Azole Antifungal Drugs against Emerging Multidrug-Resistant Candida auris. Antimicrobial Agents and Chemotherapy. 2021:65:1. https://doi.org/10.1128/AAC.00684-20
154. H. Mohammad, N. S. Abutaleb, A. M. Dieterly, L. T. Lyle, M.N. Seleem. (2021). Evaluation of ebselen in resolving a methicillin-resistant Staphylococcus aureus infection of pressure ulcers in obese and diabetic mice. PLoS ONE 16(2): e024750. https://doi.org/10.1371/journal.pone.0247508
153. Hagras M, Abutaleb NS, N. Elhosseiny, T. Abdelghany, M. N. Seleem, Mayhoub AS. (2020). Development of biphenylthiazoles exhibiting improved pharmacokinetics and potent activity against intracellular Staphylococcus aureus. 2020, 6, 11, 2887–2900. ACS Infectious Diseases IF= 4.614. https://doi.org/10.1021/acsinfecdis.0c00137
152. G. Naclerio, N. Abutaleb, D. Li, M. N. Seleem, H. Sintim.(2020). Ultrapotent inhibitor of Clostridioides difficile growth, which suppresses recurrence in vivo. 2020, 63, 20, 11934–11944. Journal of Medicinal Chemistry. IF=6.02. https://doi.org/10.1021/acs.jmedchem.0c01198
151. A. V. Morales-de-Echegaray, L. Lin, B. Sivasubramaniam, A. Yermembetova, Q. Wang, Abutaleb NS, M. N. Seleem. & A. Wei. (2020). Antimicrobial Photodynamic Activity of Gallium-Substituted Haemoglobin on Silver Nanoparticles. 2020,12, 21734-21742. Nanoscale. IF=6.97. https://doi.org/10.1039/C9NR09064A
150. H. Eldesouky, E. Salama, N. Lanman, T. Hazbun, M. N. Seleem. (2020). Potent synergistic interactions between lopinavir and azole antifungal drugs against emerging multidrug-resistant Candida auris. 10.1128/AAC.00684-20. Antimicrob Agents Chemother. IF= 4.715. https://doi.org/10.1128/AAC.00684-20
149. A. Abdelkhalek and M. N. Seleem. (2020). Repurposing the Veterinary Antiprotozoal Drug Ronidazole for the Treatment of Clostridioides difficile Infection. 2020 Oct 9;106188. International Journal of Antimicrobial Agents. IF=4.62. https://doi.org/10.1016/j.ijantimicag.2020.106188
148. J. Song, S. Malwal, N. Baig, L. Schurig-Briccio, Z. Gao, G. Vaidya, K. Yang, N. Abutaleb, M. N. Seleem, R. Gennis, T., V. Pogorelov, E. Oldfield, X. Feng. (2020). Discovery of Prenyltransferase Inhibitors with In vitro and In vivo Antibacterial Activity. 2020, 6, 11, 2979–2993. ACS Infectious Diseases IF= 4.614. https://doi.org/10.1021/acsinfecdis.0c00472
147. H. Eldesouky, N. Lanman, T. Hazbun, M. N. Seleem. (2020). Aprepitant, an antiemetic agent, interferes with metal ion homeostasis of Candida auris and displays potent synergistic interactions with azole drugs. Virulence 2020; 11(1): 1466–1481. IF=5.47. https://doi.org/10.1080/21505594.2020.1838741
146. M. G. Elnaggar, H. E. Eldesouky, Y. Pei, J. Park, H. Mohammad, Y. A. Hegazy, H. M. Tawfeek, A. A. Abdel-Rahman, A. E. Aboutaleb, M. N. Seleem, Y. Yeo. (2020). Antibacterial nanotruffles for treatment of intracellular bacterial infection. Journal: Biomaterials. August 2020, 120344. IF= 10.31. https://doi.org/10.1016/j.biomaterials.2020.120344
145. W. Zhou, A. Hsu, T. Wang, J. Jefferies, H. Mohammad, Y. Liu, Z. Luo, D. Umulis, M. N. Seleem, Q. Deng. (2020). Mitofusin 2 regulates neutrophil adhesive migration and the actin cytoskeleton. Journal of Cell Science. jcs.248880 doi: 10.1242/jcs.248880. IF= 4.57. https://doi.org/10.1242/jcs.248880
144. Kaur J, Cao X, Abutaleb NS, Elkashif A, Graboski AL, Tarawneh AH, Bhardwaj A, AbdelKhalek A, M. N. Seleem, Flaherty DP. (2020). Optimization of Acetazolamide-Based Scaffold as Potent Inhibitors of Vancomycin-Resistant Enterococcus. J. Med. Chem. Jul 8, 28, 2020. IF= 6.02. https://doi.org/10.1021/acs.jmedchem.0c00734
143. H. Hamann, Abutaleb NS, R. Pal, M. N. Seleem, P. V. Ramachandran. (2020). Gamma-Diaryl alpha-methylene-gamma-butyrolactones as potent antibacterials against methicillin-resistant Staphylococcus aureus. Bioorganic Chemistry. 28 August 2020, 104183. IF= 4.8. https://doi.org/10.1016/j.bioorg.2020.104183
142. M. Zhang, W. Hong, N. Abutaleb, J. Li, P. Dong, C. Zong, P. Wang, M. N. Seleem, Ji-Xin Cheng. (2020). Rapid Determination of Antimicrobial Susceptibility by Stimulated Raman Scattering Imaging of D2O Metabolic Incorporation in a Single Bacterium. Advanced Science. 2020,2001452. IF= 15.80. https://doi.org/10.1002/advs.202001452
141. M. Magana, M. Pushpanathan, A. Santos, L. Leanse, M. Fernandez, A. Ioannidis, M. Giulianotti, Y. Apidianakis, S. Bradfute, A. Ferguson, A. Cherkasov, M. N Seleem, C. Pinilla, C. Fuente-Nunez, T. Lazaridis, T. Dai, R. Houghten, R. Hancock, G. Tegos. (2020). The value of antimicrobial peptides in the age of resistance. The Lancet Infectious Diseases 3099(20)30327-3. IF= 71·421. https://doi.org/10.1016/S1473-3099(20)30327-3
140. N. S. Abutaleb & M. N. Seleem. (2020). Antivirulence activity and in vivo efficacy of auranofin against vancomycin-resistant enterococci (VRE). International Journal of Antimicrobial Agents. 55(3):105828 . IF= 4.6. https://doi.org/10.1016/j.ijantimicag.2019.10.009
139. A. Mancy, N. S. Abutaleb, M. Elsebaei, A. Yousef, A. Kotb, A. Ali, J. AbdelAleem, H. Mohammed, M. N. Seleem& A. Mayhoub. (2020). Balancing Physicochemical Properties of Phenylthiazole Compounds with Antibacterial Potency by Modifying the Lipophilic Side Chain. ACS Infectious Diseases. 6(1), pp. 80-90. IF= 4.91. https://doi.org/10.1021/acsinfecdis.9b00211
138. J. Hui, P. Dong, L. Liang, T. Mandal, J. Li, C. Ulloa, Y. Zhan, S. Jusuf, C. Zong, M. N. Seleem, G. Liu, Q. Cui, J. Cheng. (2020). Photo-Disassembly of Membrane Microdomains Revives Conventional Antibiotics against MRSA. Advanced Science 1903117 IF= 15.80. https://doi.org/10.1002/advs.201903117
137. V. Selvamani, A. Zareei, A. Elkashif, M. K. Maruthamuthu, S. Chittiboyina, D. Delisi, Z. Li, L. Cai, V. G. Pol, M. N. Seleem, R. Rahimi. (2020). Hierarchical Micro/Mesoporous Copper Structure with Enhanced Antimicrobial Property via Laser Surface Texturing. Advanced Material Interface. 1901890. IF= 4.85. https://doi.org/10.1002/admi.201901890
136. Hagras, M., Salama, E.A., Sayed, A.M M. N. Seleem& A. Mayhoub. (2020). Oxadiazolylthiazoles as novel and selective antifungal agents. European Journal of Medicinal Chemistry 189,112046 IF= 4.81. https://doi.org/10.1016/j.ejmech.2020.112046
135. Dokla, E.M.E., Abutaleb, N.S., Milik, S.N., Abouzid, K.A.M., M. N. Seleem. (2020). Development of benzimidazole-based derivatives as antimicrobial agents and their synergistic effect with colistin against gram-negative bacteria. European Journal of Medicinal Chemistry 186,111850 IF= 4.81. https://doi.org/10.1016/j.ejmech.2019.111850
134. Vadlamani, R.A., Dhanabal, A., Detwiler, D.A., R. Pal, M. N. Seleem., Garner, A.L. (2020). Nanosecond electric pulses rapidly enhance the inactivation of Gram-negative bacteria using Gram-positive antibiotics. Applied Microbiology and Biotechnology. Volume 104, Issue 5, 1 March 2020, Pages 2217-2227. IF= 3.67. https://doi.org/10.1007/s00253-020-10365-w
133. Naclerio GA, Abutaleb NS, Onyedibe KI, M. N. Seleemand Sintim HO. (2020). Potent trifluoromethoxy, trifluoromethylsulfonyl, trifluoromethylthio and pentafluorosulfanyl containing (1,3,4-oxadiazol-2-yl) benzamides against drug-resistant Gram-positive bacteria. RSC Med. Chem. 2020,11, 102-110 IF= 3.049 https://doi.org/10.1039/C9MD00391F
132. Hammad S., Abutaleb NS, D. Li, I. Ramming, A. Shekhar, Abdel-Halim M., E. Elrazaz, M. N. Seleem, U. Bilitewski, K. Abouzid, E. El-Hossary. (2020). Synthesis and Antimicrobial evaluation of New Halogenated 1,3-Thiazolidin-4-ones. Bioorg Chem., 103517. IF= 3.9. https://doi.org/10.1016/j.bioorg.2019.103517
131. Hosny Y, Abutaleb NS, Omara M, Alhashimi M, Elsebaei MM, Elzahabi HS, N. Seleem & A. Mayhoub. (2020). Modifying the lipophilic part of phenylthiazole antibiotics to control their drug-likeness. Eur J Med Chem 2020, 185:111830. IF= 4.83. https://doi.org/10.1016/j.ejmech.2019.111830
130. H. Eldesouky, E. Salam, L. Xiaoyan, H. Mohammad, T. Hazbun, A. Mayhoub & M. N. Seleem. (2020). Repurposing approach identifies pitavastatin as a potent azole chemosensitizing agent effective against azole-resistant Candida species. Scientific reports 10 (1), 1-12 IF= 4.25. https://doi.org/10.1038/s41598-020-64571-7
129. C. Ghosh, A. AbdelKhalek, H. Mohammad, M. N. Seleem& J. Haldar. (2020) Aryl-alkyl-lysines: Novel agents for treatment of C. difficile infection. Scientific reports 10 (1), 1-7 IF= 4.25. https://doi.org/10.1038/s41598-020-62496-9
128. A. Abdelkhalek, H. Mohammad, A. Mayhoub & M. N. Seleem. (2020). Screening for potent and selective anticlostridial leads among FDA-approved drugs. The Journal of Antibiotics 73 (6), 392-409. IF= 2.60. https://doi.org/10.1038/s41429-020-0288-3
127. Young Jin Seong, A. Mayhoub, H. Mohammad & M. N. Seleem. (2020). Repurposing fenamic acids drugs to combat multidrug-resistant Neisseria gonorrhoeae. Antimicrobial Agents and Chemotherapy. 10.1128/AAC.02206-19. IF= 4.60. https://doi.org/10.1128/AAC.02206-19
126. H. Eldesouky, E. Salam, T. Hazbun, A. Mayhoub & M. N. Seleem. (2020). Ospemifene displays broad-spectrum synergistic interactions with itraconazole through potent interference with fungal efflux activities. Scientific reports 10 (6089) IF= 4.25. https://doi.org/10.1038/s41598-020-62976-y
125. Abutaleb NS, M. N. Seleem. (2020). Repurposing the antiamoebic drug diiodohydroxyquinoline for treatment of Clostridioides difficile infections. Antimicrob Agents Chemother. 64 (6) IF= 4.715. https://doi.org/10.1128/AAC.02115-19
124. R. Pal and M. N. Seleem(2020). Screening of Natural Products and Approved Oncology Drug Libraries for Activity against Clostridioides difficile. Scientific Reports. 10 (1), 1-8. IF= 4.25. https://doi.org/10.1038/s41598-020-63029-0
123. Mohammad, H., N. Abutaleb, M. N. Seleem. (2020). Auranofin Rapidly Eradicates Methicillin-resistant Staphylococcus aureus (MRSA) in an Infected Pressure Ulcer Mouse Model. Scientific Reports. 10 (1), 1-8 IF= 4.25. https://doi.org/10.1038/s41598-020-64352-2
122. A. Elkashif and M. N. Seleem. (2020). Investigation of auranofin and gold-containing analogues antibacterial activity against multidrug-resistant Neisseria gonorrhoeae. Scientific Reports. 10 (1), 1-9. IF= 4.25. https://doi.org/10.1038/s41598-020-62696-3
121. Thomas A. Dietsche, Eldesouky HE, M. N. Seleem, and Jean Chmielewski (2020). Targeting Intracellular Pathogenic Bacteria Through N-Terminal Modification of Cationic Amphiphilic Polyproline Helices. The Journal of Organic Chemistry. 2020, 85, 11, 7468–7475. IF= 4.8. https://doi.org/10.1021/acs.joc.0c00871
120. Abutaleb NS, M. N. Seleem. (2020). Auranofin, at clinically achievable dose, protects mice and prevents recurrence from Clostridioides difficile infection. Scientific Reports 10 (1), 1-8. IF= 4.25. https://doi.org/10.1038/s41598-020-64882-9
119. I. Shahin, Abutaleb NS, M. Alhashimi, A. Kassab, K..Mohamed, A. Taher, N. Seleem & A. Mayhoub. (2020). Evaluation of N-Phenyl-2-aminothiazoles for Treatment of Multi-Drug Resistant and Intracellular Staphylococcus aureus Infections. Eur J Med Chem 2020, 112497. IF= 4.83. https://doi.org/10.1016/j.ejmech.2020.112497
118. A Roth, A. Elkashif, V. Selvamani, M. N. Seleem, B. Ziaie, R. Rahim. (2020). Wearable and Flexible Ozone Generating System for Treatment of Infected Dermal Wounds. Frontiers in Bioengineering and Biotechnology 8, 458. IF= 5.12. https://doi.org/10.3389/fbioe.2020.00458
117. A. Abdelkhalek, N. Abutaleb, H. Mohammad & M. N. Seleem. (2019). Antibacterial and antivirulence activities of auranofin against Clostridium difficile. International Journal of Antimicrobial Agents 53: 54-62. IF= 4.30. https://doi.org/10.1016/j.ijantimicag.2018.09.018
116. S. Malwal, L. Chen, H. Hicks, F. Qu, W. Liu, A. Shillo, W. Law, J. Zhang, N. Chandnani, X. Han, Y. Zheng, C. Chen, R. Guo, A. AbdelKhalek, M. N. Seleem & E. Oldfield. (2019) Discovery of lipophilic bisphosphonates that target bacterial cell wall and quinone biosynthesis. Journal of Medicinal Chemistry. 62 (5), 2564-2581. IF= 6.25. https://doi.org/10.1021/acs.jmedchem.8b01878
115. A. Kotb, N. S. Abutaleb, M. Hagras, A. Bayoumi, M. Mohamed, A. Moustafa, A. Ghiaty, M. N. Seleem& A. Mayhoub. (2019). tert-Butylphenylthiazoles with oxadiazole linker: A novel orally bioavailable class of antibiotics exhibiting antibiofilm activity. RSC Advances. 9 (12), 6770-6778. IF= 2.93. https://doi.org/10.1039/C8RA10525A
114. P. Dong, H. Mohammad, X. Wang, J. Hui, J. Li, L. Liang, M. N. Seleem& Ji-Xin Cheng. (2019). Photolysis of staphyloxanthin in methicillin-resistant Staphylococcus aureus potentiates killing by reactive oxygen species. Advanced Science. 2019, 1900030. IF= 15.80. https://doi.org/10.1002/advs.201900030
113. K. Kyei-Baffoura, H. Mohammad, M. N. Seleem& M. Dai. (2019). Second-generation aryl isonitrile compounds targeting multidrug-resistant Staphylococcus aureus. Bioorganic & Medicinal Chemistry. 27 (9), 1845-1854. IF= 2.9. https://doi.org/10.1016/j.bmc.2019.03.034
112. M. Elsebaei, H. Mohammad, N. S. Abutaleb, A. Samir, A. Norvil, A. Michie, M. Gad, H. Gowher, M. N. Seleem& A. Mayhoub. (2019). Lipophilic efficient phenylthiazoles with potent undecaprenyl pyrophosphatase inhibitory activity. European Journal of Medicinal Chemistry. 175, 49-62. IF= 4.81. https://doi.org/10.1016/j.ejmech.2019.04.063
111. H. Mohammad, K. Kyei-Baffoura, N. Abutaleb, M. Dai & M. N. Seleem(2019). An aryl isonitrile compound with an improved physicochemical profile that is effective in two mouse models of multidrug-resistant Staphylococcus aureus infection. Journal of global antimicrobial resistance. S2213-7165(19)30105-5. IF= 2.46. https://doi.org/10.1016/j.jgar.2019.04.016
110. B. Sloan, H. Mohammad, N. S. Abutaleb & M. N. Seleem. (2019). Comparison between a novel tap water wound irrigation device and a sterile saline device in an animal model. Trauma. 2019-3-1-6. https://doi.org/10.1177/1460408619857399
109. M. Elsebaei, N. Abutaleb, Abdulrahman Mahgoub, D. Li, M. Hagras, H. Mohammad, M. N. Seleem& A. Mayhoub. (2019). Phenylthiazoles with Nitrogenous Side Chain: An Approach to Overcome Molecular Obesity. European Journal of Medicinal Chemistry. 19-01362. IF= 4.81. https://doi.org/10.1016/j.ejmech.2019.111593
108. X. Shao, A. Abdelkhalek, N. Abutaleb, U. Velagapudi M. N. Seleem& T. Talele. (2019). Chemical space exploration around thieno[3,2-d]pyrimidin-4(3H)-one scaffold led to a novel class of highly active Clostridium difficile inhibitors. Journal of Medicinal Chemistry. 62(21), pp. 9772-9791. IF= 6.25. https://doi.org/10.1021/acs.jmedchem.9b01198
107. A. Hammad, N. S. Abutaleb, M. Elsebaei, M. Ali, M. Al-Sawah, M. N. Seleem& A. Mayhoub. (2019). From phenylthiazoles to phenylpyrazoles: Broaden the antibacterial spectrum towards carbapenam-resistant strains. Journal of Medicinal Chemistry. 62(17), pp. 7998-8010 IF= 6.25. https://doi.org/10.1021/acs.jmedchem.9b00720
106. N. Dayal, C. Opoku-Temeng, H. Mohammad, N. S. Abutaleb, D. Hernandez, M. N. Seleem& H. Sintim. (2019). Inhibitors of Intracellular Gram-Positive Bacterial Growth Synthesized via Povarov‚àíDoebner Reactions. ACS Infectious Diseases. 5(11), pp. 1820-1830. IF= 4.91. https://doi.org/10.1021/acsinfecdis.9b00022
105. S. Al-Trawneh, N. Abutaleb, E. Salama, A. Tarawneh, and M. N. Seleem. (2019). Synthesis of New Pyrazolo Triazines with antifungal antibiofilm activity. Medicinal Chemistry Research. MCRE-D-19-00300 IF= 1.7. https://doi.org/10.1007/s11696-019-00974-9
104. D. Mody, A. Athamneh, & M. N. Seleem. (2019). Curcumin: A natural derivative with antibacterial activity against Clostridium difficile. Journal of Global Antimicrobial Resistance. JGAR-D-19-00442. IF= 2.9. https://doi.org/10.1016/j.jgar.2019.10.005
103. M. Alhashimi & M. N. Seleem. (2019). Repurposing salicylamide for combating multidrug-resistant Neisseria gonorrhoeae. Antimicrobial Agents and Chemotherapy 63(12),e01225-19. IF= 4.7. https://doi.org/10.1128/AAC.01225-19
102. Mohammad, H., Eldesouky, H.E., Hazbun, T., Mayhoub, A.S., M. N. Seleem. (2019). Identification of a Phenylthiazole Small Molecule with Dual Antifungal and Antibiofilm Activity Against Candida albicans and Candida auris. Scientific Reports 9(1),18941 IF= 4.25. https://doi.org/10.1038/s41598-019-55379-1
101. M. Hagras, Y. Hegazy, A. Elkabbany, H. Mohammad, A. Ghiaty, T. Abdelgahny & M. N. Seleem& A. Mayhoub. (2018). Biphenylthiazole antibiotics with an oxadiazole linker: An approach to improve physicochemical properties and oral bioavailability. European Journal of Medicinal Chemistry. 143: 1448-1456. IF= 4.81. https://doi.org/10.1016/j.ejmech.2017.10.048
100. H. Eldesouky, A. Mayhoub, T. R. Hazbun & M. N. Seleem. (2018). Reversal of azole resistance in Candida albicans by sulfa antibacterial drugs. Antimicrobial Agents and Chemotherapy 62 (3): e00701-17. IF= 4.45. https://doi.org/10.1128/AAC.00701-17
99. H. Mohammad, A. Abdelkhalek, N. Abutaleb & M. N. Seleem. (2018). Evaluation of the anthelmintic drug niclosamide for intestinal decolonization of vancomycin-resistant Enterococci. International Journal of Antimicrobial Agents 51 (6): 897-904. IF= 4.30. https://doi.org/10.1016/j.ijantimicag.2018.02.003
98. W. Hong, C. W. Karanja, W. Younis, X. Zhang, M. N. Seleem& Ji-Xin Cheng. (2018). Antibiotic susceptibility determination within one cell cycle at single bacterium level by stimulated Raman metabolic imaging. Analytical Chemistry 90(6):3737-3743 IF= 6.32. https://doi.org/10.1021/acs.analchem.7b03382
97. H. Mohammad, N. ElGhazawy, H. Eldesouky, Y. Hegazy, W. Younis, L. Avrimova, T. Hazbun, R. Arafa & M. N. Seleem. (2018). Discovery of a novel dibromoquinoline compound exhibiting potent antifungal and antivirulence activity that targets metal ion homeostasis. ACS Infectious Diseases. 4 (3): 403-414 IF= 4.91. https://doi.org/10.1021/acsinfecdis.7b00215
96. M. M. Elsebaei, H. Mohammad, M. Abouf, N. Abutaleb, Y. Hegazy, A. Ghiaty, L. Chen, J. Zhang, E. Oldfield, M. N. Seleem& A. Mayhoub.(2018). Alkynyl-containing phenylthiazoles: Systemically active antibacterial agents effective against methicillin-resistant Staphylococcus aureus (MRSA). European Journal of Medicinal Chemistry 148: 195-209. IF= 4.81. https://doi.org/10.1016/j.ejmech.2018.02.031
95. B. E. Bergstrom, A. Abdelkhalek, W. Younis, G. K. Hammac, W. M. Townsend & M. N. Seleem. (2018). Antibacterial activity and safety of commercial veterinary cationic steroid antibiotics and neutral superoxidized water. PloS One 13(3):e0193217. IF= 2.8. https://doi.org/10.1371/journal.pone.0193217
94. C. Opoku-Temeng, H. Mohammad, N. Dayal, G. Naclerio, N. Abutaleb , M. N. Seleem& H. Sintim. (2018). N-(1,3,4-oxadiazol-2-yl)benzamide analogs, bacteriostatic agents against methicillin- and vancomycin-resistant bacteria. European Journal of Medicinal Chemistry. 155: 797-805. IF= 4.81. https://doi.org/10.1016/j.ejmech.2018.06.023
93. A. Kotb, N. Abutaleb, M. Seleem, M. Hagras, H. Mohammad, A. Bayoumi, A. Ghiaty, M. N. Seleem& A. Mayhoub.(2018). Phenylthiazoles with tert-Butyl Side Chain: Metabolically stable with anti-biofilm activity. European Journal of Medicinal Chemistry. 151; 110-120. IF= 4.81 . https://doi.org/10.1016/j.ejmech.2018.03.044
92. M. Awamy, H. Mohammad, A. Hussien, N. Abutaleb, M. Hagras, R. Serya, A. Taher, K. Abuzaid, M. N. Seleem& A. Mayhoub.(2018). Alkoxyphenylthiazoles with broad-spectrum activity against multidrug-resistant Gram-positive bacterial pathogens. European Journal of Medicinal Chemistry. 152: 318-328. IF= 4.81. https://doi.org/10.1016/j.ejmech.2018.04.049
91. A. Abdelkhalek, N. Abutaleb, K. Elmagarmid & M. N. Seleem. (2018). Repurposing auranofin as an intestinal decolonizing agent for vancomycin-resistant Enterococci. Scientific Reports 8 (1): 8353 IF= 4.25. https://doi.org/10.1038/s41598-018-26674-0
90. M. Nepal, M. Mohamed, R. Blade, H. Eldesouky, T. Anderson, M. N. Seleem& J. Chmielewski (2018). A library approach to cationic amphiphilic polyproline helices that target intracellular pathogenic bacteria. ACS Infectious Diseases 4; 1300-1305. IF= 4.91. https://doi.org/10.1021/acsinfecdis.8b00124
89. A. Abdelkhalek, N. Abutaleb, H. Mohammad & M. N. Seleem. (2018). Repurposing ebselen for decolonization of vancomycin-resistant enterococci (VRE). PloS One 13 (6): e0199710 IF= 2.8. https://doi.org/10.1371/journal.pone.0199710
88. H. Eldesouky, L. Xiaoyan, N. Abutaleb, H. Mohammad & M. N. Seleem. (2018). Synergistic interactions of sulfamethoxazole and azole antifungal drugs against emerging multidrug-resistant Candida auris. International Journal of Antimicrobial Agents. 52 (6): 754-761 IF= 4.30. https://doi.org/10.1016/j.ijantimicag.2018.08.016
87. A. Morales, T. Maltais, L. Lu, W. Younis, N. Kadasala, M. N. Seleem& A. Wei. (2018). Rapid uptake and photodynamic inactivation of Staphylococci by ga(III)-protoporphyrin IX. ACS Infectious Diseases 11; 1564-1573. IF= 4.82. https://doi.org/10.1021/acsinfecdis.8b00125
86. E. Yahia, H. Mohammad, T. Abdelghany, E. Fayed, M. N. Seleem& A. Mayhoub. (2017). Phenylthiazole antibiotics: A metabolism-guided approach to overcome short duration of action. European Journal of Medicinal Chemistry 126: 604-61. IF= 4.81. https://doi.org/10.1016/j.ejmech.2016.11.042
85. S. Thangamani, M. Maland, H. Mohammad, P. Pascuzzi , L. Avramova, C. Koehler, T. Hazbun & M. N. Seleem. (2017). Repurposing approach identifies auranofin with broad spectrum antifungal activity that targets Mia40-Erv1 pathway. Frontiers in Cellular and Infection Microbiology 7:4. IF= 4.3. https://doi.org/10.3389/fcimb.2017.00004
84. W. Younis, A. AbdelKhalek, A. Mayhoub & M. N. Seleem. (2017). In vitro screening of an FDA-approved library against ESKAPE pathogens. Current pharmaceutical design 23(14):2147-2157. IF= 3.052. http://doi.org/10.2174/1381612823666170209154745
83. C. Ghosh, V. Yadav, W. Younis, H. Mohammad, Y. Hegazy, M.N. Seleem, K. Sanyal & J. Haldar. (2017). Aryl-alkyl-lysines: Membrane-active fungicides that act against biofilms of Candida albicans. ACS Infectious Diseases 3(4):293-301. IF= 4.91. https://doi.org/10.1021/acsinfecdis.6b00192
82. I. Eissa, H. Mohammad, O. Qassem, W. Younis, T. Abdelghany, A. Elshafeey, M. Moustafa, M.N. Seleem & A. Mayhoub. (2017). Diphenylurea derivatives for combating methicillin-and vancomycin-resistant Staphylococcus aureus. European Journal of Medicinal Chemistry 130; 73-85. IF= 4.81. https://doi.org/10.1016/j.ejmech.2017.02.044
81. H. Mohammad, K. Kyei-Baffourb, W. Younis, D. Davis, H. Eldesouky, M. N. Seleem& M. Dai. (2017). Investigation of aryl isonitrile compounds with potent, broad-spectrum antifungal activity. Bioorganic & Medicinal Chemistry 25:2926–2931. IF= 3.79. https://doi.org/10.1016/j.bmc.2017.03.035
80. S. Thangamani, H. Eldesouky, H. Mohammad, P. Pascuzzi, L. Avramova, T. Hazbun & M.N. Seleem. (2017). Ebselen exerts antifungal activity by regulating glutathione (GSH) and reactive oxygen species (ROS) production in fungal cells. Biochimica et Biophysica Acta (BBA)-General Subjects 1861 (1): 3002-3010. IF= 4.70. https://doi.org/10.1016/j.bbagen.2016.09.029
79. M. Mohamed, A. Brezden, H. Mohammad, J. Chmielewski & M.N. Seleem. (2017). Targeting biofilms and persisters of ESKAPE pathogens with P14KanS, a kanamycin peptide conjugate. Biochimica et Biophysica Acta (BBA)-General Subjects 1861 (4): 848-859. IF= 4.70. https://doi.org/10.1016/j.bbagen.2017.01.029
78. H. Mohammad, W. Younis, L. Chen, C. Peters, J. Pogliano, K. Pogliano, B. Cooper, J. Zhang, A. Mayhoub, E. Oldfield, M. Cushman & M.N. Seleem. (2017). Phenylthiazole antibacterial agents targeting cell wall synthesis exhibit potent activity in vitro and in vivo against vancomycin-resistant enterococci. Journal of Medicinal Chemistry 60(6):2425-2438. IF= 6.25. https://doi.org/10.1021/acs.jmedchem.6b01780
77. M. Hagras, H. Mohammad, M. Mandour, Y. Hegzy, A. Ghiaty, M. N. Seleem& A. Mayhoub. (2017). Investigating the antibacterial activity of biphenylthiazoles against methicillin- and vancomycin-resistant Staphylococcus aureus (MRSA and VRSA). Journal of Medicinal Chemistry. 60 (9): 4074–4085. IF= 6.25. https://doi.org/10.1021/acs.jmedchem.7b00392
76. X. Yin, H. Mohammed, H. Eldesouky, A. Hassan, M. N. Seleem& M. Dai. (2017). Rapid synthesis of vicyclic lactones via palladium-catalyzed aminocarbonylative lactonizations. Chemical Communications. 53;7238-7241. IF= 6.319. https://doi.org/10.1039/C7CC02494K
75. M. Mohamed, A. Brezden, H. Mohammad, J. Chmielewski & M.N. Seleem. (2017). A short D-enantiomeric antimicrobial peptide with potent immunomodulatory and antibiofilm activity against multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Scientific Reports 7: 6953. IF= 4.25. https://doi.org/10.1038/s41598-017-07440-0
74. H. Hussain, Z. Iqbal, M. N. Seleem, D. Huang, A. Sattar, H. Hao & Z. Yuan. (2017). Virulence and transcriptome profile of multidrug-resistant Escherichia coli from chicken. Scientific Reports. 7: 8335. IF= 4.25. https://doi.org/10.1038/s41598-017-07798-1
73. Y. Pei, M. F. Mohamed, M. N. Seleem& Y. Yeo. (2017). Particle engineering for intracellular delivery of vancomycin to methicillin-resistant Staphylococcus aureus (MRSA)-infected macrophages. The Journal of Controlled Release. 267; 133-143. IF= 7.87 . https://doi.org/10.1016/j.jconrel.2017.08.007
72. H. Mohammad, W. Younis, H. Ezzat, C. Peters, A. Abdelkhalek, J. Pogliano, K. Pogliano, B. Cooper, A. Mayhoub & M. N. Seleem. (2017). Diphenylurea compounds targeting bacterial cell wall synthesis exhibit potent antibacterial activity. PLoS One. 12(8):e0182821. IF= 2.8. https://doi.org/10.1371/journal.pone.0182821
71. M. I. Hamed, T. L. McCalla, W. M. Townsend & M. N. Seleem. (2017). Staphylococcus pseudintermedius Isolated from Two Dog Cases with Ophthalmic Lesions. American Journal of Infectious Diseases and Microbiology, 5(4): 132-136. http://pubs.sciepub.com/ajidm/5/4/2
70. C. W. Karanja, W. Hong, W. Younis, H. Eldesouky, M. N. Seleem& Ji-Xin Cheng. (2017). Stimulated Raman imaging reveals aberrant lipogenesis as a metabolic marker for azole-resistant Candida albicans. Analytical Chemistry. 18; 9822-9829. IF= 6.32. https://doi.org/10.1021/acs.analchem.7b01798
69. I. Eid, M. Elsebaei, H. Mohammad, M. Hagras, C. E. Peters, Y. Hegazy, B. Cooper, J. Pogliano, K. Pogliano, H. S. Abulkhair, M. N. Seleem& A. Mayhoub. (2017). Arylthiazole Antibiotics Targeting Intracellular Methicillin-resistant Staphylococcus aureus (MRSA) Interfere with Bacterial Cell Wall Synthesis. European Journal of Medicinal Chemistry.139; 665-673. IF= 4.81. https://doi.org/10.1016/j.ejmech.2017.08.039
68. M. Abushahba, H. Mohammad, S. Thangamani, A. Hussein & M. N. Seleem. (2016). Impact of different cell penetrating peptides on the efficacy of antisense therapeutics for targeting intracellular pathogens. Scientific Reports 6: 20832. IF= 4.25. https://doi.org/10.1038/srep20832
67. S. Thangamani, H. Mohammad, M. Abushahba, T. Sobreira & M. N. Seleem. (2016). Repurposing auranofin for the treatment of cutaneous staphylococcal infections. International journal of antimicrobial agents 47 (3): 195-201. IF= 4.30. https://doi.org/10.1016/j.ijantimicag.2015.12.016
66. S. Thangamani, H. Mohammad, M. Abushahba, T. Sobreira, V Hedrick, L. Paul & M. N. Seleem. (2016). Antibacterial activity and mechanism of action of auranofin against multi-drug resistant bacterial pathogens. Scientific Reports 6: 22571. IF= 4.25. https://doi.org/10.1038/srep22571
65. W. Hong, C. Liao, H. Zhao, W. Younis, Y. Zhang, M. N. Seleem& J. Cheng. (2016). In situ detection of a single bacterium in complex environment by hyperspectral CARS imaging. ChemistrySelect 3: 513–517 IF 1.505 . https://doi.org/10.1002/slct.201600166
64. M. Seleem, A. Disouky, H. Mohammad, T. Abdelghany, A. Mancy, S. Bayoumi, A. Elshafeey, A. El-Morsy, M. N. Seleem& A. S. Mayhoub. (2016). Second-generation phenylthiazole antibiotics with enhanced pharmacokinetic properties. Journal of Medicinal Chemistry 59(10):4900-12. IF= 6.25. https://doi.org/10.1021/acs.jmedchem.6b00233
63. M. Abushahba, H. Mohammad & M. N. Seleem(2016). Targeting multidrug-resistant Staphylococci with an anti-rpoA peptide nucleic acid conjugated to the HIV-1 TAT cell penetrating peptide. Molecular Therapy—Nucleic Acids 5 (7): e339. IF= 6.39. https://doi.org/10.1038/mtna.2016.53
62. M. Abushahba, A. Hussein, M. N. Seleem& R Hassanein. (2016). Listeria monocytogenes: Overview and Targeting Advances. Journal of Advanced Veterinary Research 6 (2): 72-80. https://advetresearch.com/index.php/AVR/article/view/30/26
61. T. Maltais, A. Adak, W. Younis, M. N. Seleem& A. Wei. (2016). Label-Free detection and discrimination of bacterial pathogens based on hemin recognition. Bioconjugate Chemistry 27 (7): 1713–1722. IF= 4.81. https://doi.org/10.1021/acs.bioconjchem.6b00236
60. A. AbdelKhalek, C. Ashby, B. Patel, T. Talele & M. N. Seleem. (2016). In vitro antibacterial activity of rhodanine derivatives against pathogenic clinical isolates. PLoS One 11(10):e0164227. IF= 2.8. https://doi.org/10.1371/journal.pone.0164227
59. A. Brezden, M. Mohamed, M. Nepal, J. Harwood, J. Kuriakose, M. N. Seleem& J. Chmielewski. (2016). Dual targeting of intracellular pathogenic bacteria with a cleavable conjugate of kanamycin and an antibacterial, cell penetrating peptide. Journal of the American Chemical Society 138 (34): 10945–10949. IF= 14.35. https://doi.org/10.1021/jacs.6b04831
58. W. Younis, H. Mohammad, M. Hostetler, D. López-Pérez, C. Steussy, M. Lipton, C. Stauffacher, S. Sultan, M. Wael, A. Hussein & M. N. Seleem. (2016). Class II HMG-CoA reductase inhibitors targeting methicillin-resistant Staphylococcus pseudintermedius. Journal of Advanced Veterinary Research (7)1: 1-6. https://advetresearch.com/index.php/AVR/article/view/3/3
57. Z. Iqbal, M. N. Seleem, H. Iftikhar H. Huang, H. Hao & Z. Yuan. (2016). Comparative virulence studies and transcriptome analysis of Staphylococcus aureus strains isolated from animals. Scientific Reports 6: 35442. IF= 4.25. https://doi.org/10.1038/srep35442
56. M. Mohamed, A. Abdelkhalek & M. N. Seleem. (2016). Evaluation of short synthetic antimicrobial peptides for treatment of drug-resistant and intracellular Staphylococcus aureus. Scientific Reports 6: 29707. IF= 4.25. https://doi.org/10.1038/srep29707
55. S. Thangamani, H. Mohammad, M. Abushahba, M. Hamed, T. Sobreira, V. Hedrick, L. Paul & M. N. Seleem. (2015). Exploring simvastatin, an antihyperlipidemic drug, as a potential topical antibacterial agent. Scientific Reports 5: 16407. IF= 4.25. https://doi.org/10.1038/srep16407
54. H. Mohammad, M. Cushman & M. N. Seleem. (2015). Antibacterial evaluation of synthetic thiazole compounds in vitro and in vivo in a methicillin-resistant Staphylococcus aureus (MRSA) Skin Infection Mouse Model. PLoS One 10(11):e0142321. IF= 2.8. https://doi.org/10.1371/journal.pone.0142321
53. S. Thangamani, M. Nepal, J. Chmielewsk & M. N. Seleem. (2015). Antibacterial activity and therapeutic efficacy of Fl-PRPRPL-5, a cationic amphiphilic polyproline helix, in a mouse model of staphylococcal skin infection. Drug Design, Development and Therapy 9: 5749—5754. IF= 3.02. https://doi.org/10.2147/DDDT.S94505
52. S. Thangamani, W. Younis & M. N. Seleem. (2015). Repurposing celecoxib as a topical antimicrobial agent. Frontiers Microbiology 28: 6:750. IF= 4.16. https://doi.org/10.3389/fmicb.2015.00750
51. S. Thangamani, W. Younis & M. N. Seleem. (2015). Repurposing clinical molecule ebselen to combat drug resistant pathogens. PLoS One 10 (7):e0133877. IF= 2.8. https://doi.org/10.1038/srep11596
50. W. Younis, S. Thangamani & M. N. Seleem. (2015). Repurposing Non-antimicrobial Drugs and Clinical Molecules to Treat Bacterial Infections. Current Pharmaceutical Design 21(28):4106-11. IF= 3.052. http://doi.org/10.2174/1381612821666150506154434
49. Dabral N, Jain-Gupta N, M. N. Seleem, Sriranganathan N & Vemulapalli R. (2015). Overexpression of Brucella putative glycosyltransferase WbkA in Brucella abortus RB51 leads to production of exopolysaccharide. Frontiers in Cellular and Infection Microbiology 24;5:54. IF= 4.3. https://doi.org/10.3389/fcimb.2015.00054
48. Davis DC, Mohammad H, Kyei-Baffour K, Younis W, Creemer CN, M. N. Seleem& Dai M. (2015). Discovery and characterization of aryl isonitriles as a new class of compounds versus methicillin- and vancomycin-resistant Staphylococcus aureus. European Journal of Medicinal Chemistry 101:384-390. IF= 4.81. https://doi.org/10.1016/j.ejmech.2015.06.031
47. S. Thangamani, W. Younis & M. N. Seleem. (2015). Repurposing ebselen for treatment of multidrug-resistant staphylococcal infections. Scientific Reports 5:11596. IF= 4.25. https://doi.org/10.1038/srep11596
46. M. Nepal, S. Thangamani, M. N. Seleem& J. Chmielewski. (2015). Targeting intracellular bacteria with an extended cationic amphiphilic polyproline helix. Organic & Biomolecular Chemistry 13 (21):5930-6. IF= 3.56. https://doi.org/10.1039/C5OB00227C
45. H. Mohammad, PV Reddy, D. Montelenoe, AS Mayhoub, M. Cushman, GK Hammac & M. N. Seleem. (2015). Antibacterial Characterization of Novel Synthetic Thiazole compounds against methicillin-resistant Staphylococcus pseudintermedius. PLoS One 10(6):e0130385. IF= 2.8. https://doi.org/10.1371/journal.pone.0130385
44. Mohammad, H., Reddy, P.V.N., Monteleone, D., Cushman, M., & M. N. Seleem. (2015). Synthesis and antibacterial evaluation of a novel series of synthetic phenylthiazole compounds against methicillin-resistant Staphylococcus aureus (MRSA). European Journal of Medicinal Chemistry 94:306-316. IF= 4.81. https://doi.org/10.1016/j.ejmech.2015.03.015
43. H. Mohammad, S. Thangamani & M. N. Seleem. (2015). Antimicrobial peptides and peptidomimetics-potent therapeutic allies for staphylococcal infections. Current Pharmaceutical Design 21 (16): 2073-2088. IF= 3.052 (invited review article) . http://doi.org/10.2174/1381612821666150310102702
42. S. Thangamani, H. Mohammad, W. Younis & M. N. Seleem. (2015). Repurposing non-antimicrobial drugs for treatment of staphylococcal infections. Current Pharmaceutical Design 21 (16): 2089-2100. IF= 3.052 (invited review article). http://doi.org/10.2174/1381612821666150310104416
41. H. Mohammad, A. S. Mayhoub, M. Cushman & M. N. Seleem. (2015). Anti-biofilm activity and synergism of novel thiazole compounds with glycopeptide antibiotics against multidrug-resistant staphylococci. Nature Journal of Antibiotics 68(4):259-66. IF= 2.23. https://doi.org/10.1038/ja.2014.142
40. E. A. Swanson, L. J. Freeman, M. N. Seleem& P. W. Snyder. (2014). Biofilm-infected wounds in a dog: Case report and review of the literature. Journal of the American Veterinary Medical Association 244(6): 699-707. IF=1.67 . https://doi.org/10.2460/javma.244.6.699
39. W. Lv, B. Banerjee, K. Molland, M. N. Seleem, A. Ghafoor, M. Hamed, B. Wan, S. Franzblau & A. Mesecar, M. Cushman. (2014). Synthesis and Antibacterial Activity of 3-(3-Aryl-pyrrolidin-1-yl)-5-aryl-1,2,4-triazine Inorganic Pyrophosphatase Inhibitors. Bioorganic & Medicinal Chemistry 22 (1): 406-18. IF=2.95. https://doi.org/10.1016/j.bmc.2013.11.011
38. H. Mohammad, A. S. Mayhoub, A. Ghafor, M. Soofi, R. Alajlouni, M. Cushman & M. N. Seleem. (2014). Discovery and characterization of potent substituted thiazole compounds versus methicillin-and vancomycin-resistant Staphylococcus aureus. Journal of Medicinal Chemistry 57 (4): 1609–1615. IF= 6.25. https://doi.org/10.1021/jm401905m
37. A. Athamneh, R. Alajlouni, R. S. Wallace, M. N. Seleem& Ryan Senger. (2014). Phenotypic profiling of antibiotic response signatures in Escherichia coli using Raman spectroscopy. Antimicrobial Agents and Chemotherapy 58 (3): 1302–1314. IF=4.45 . https://doi.org/10.1128/AAC.02098-13
36. M. F. Mohamed, M. I. Hamed, A. Panitch & M. N. Seleem. (2014).Targeting methicillin-resistant Staphylococcus aureus with short synthetic peptides: coupling membrane disruption with DNA binding. Antimicrobial Agents and Chemotherapy 58 (7): 4113-4122. IF= 4.45 . https://doi.org/10.1128/AAC.02578-14
35. M. F. Mohamed & M. N. Seleem. (2014). Efficacy of short novel antimicrobial and anti-inflammatory peptides in a mouse model of methicillin-resistant Staphylococcus aureus (MRSA) skin infection. Drug Design, Development and Therapy 8: 1979-1983. IF= 3.02. https://doi.org/10.2147/DDDT.S72129
34. M. F. Mohamed, G. K. Hammac, L. Guptill & M. N. Seleem. (2014). Antibacterial activity of novel cationic peptides against clinical isolates of multi-drug resistant staphylococcus pseudintermedius from infected dogs. PLoS One 9(12):e116259. IF= 2.80. https://doi.org/10.1371/journal.pone.0116259
33. N. Jain, A. Contreras, G. Kimswade, M. N. Seleem, S. M. Boyle & N. Sriranganathan. (2011). Effect of entF deletion on iron acquisition and erythritol metabolism by Brucella abortus 2308. FEMS Microbiology Letters 316 (1):1-6. IF= 2.72. https://doi.org/10.1111/j.1574-6968.2010.02186.x
32. P. Rajasekaran, N. Surendran, M. N. Seleem, N. Sriranganathan, G. G. Schurig & S. M. Boyle. (2011). Over-expression of homologous antigens in a leucine auxotroph of Brucella abortus strain RB51 protects mice against a virulent Brucella suis challenge. Vaccine 29 (17):3106-3110. IF= 3.48. https://doi.org/10.1016/j.vaccine.2011.02.054
31. A. Ranjan, N. Pothayee, M. N. Seleem, S. M. Boyle, R. Kasimanickam, J. S. Riffle &N. Sriranganathan . (2012). Nanomedicine for intracellular therapy. FEMS Microbiology letters 332 (1):1-9. IF=2.72. https://doi.org/10.1111/j.1574-6968.2012.02566.x
30. M. Soofi & M. N. Seleem. (2012). Targeting Salmonella essential genes with antisense peptide nucleic acid. Antimicrobial Agents and Chemotherapy 56 (12): 6407-9. IF= 4.45 . https://doi.org/10.1128/AAC.01437-12
29. P. Rajesekaran, J. Alexander, M. N. Seleem, N. Jain, N. Sriranganathan, A. Wattam, J. Setubal & S. M. Boyle. (2013). Peptide nucleic acids inhibit Brucella suis in pure culture and infected macrophages. International Journal of Antimicrobial Agents 41 (4): 358-62. IF= 4.3. https://doi.org/10.1016/j.ijantimicag.2012.11.017
28. R. Alajlouni & M. N. Seleem. (2013). Targeting Listeria monocytogenes rpoA and rpoD genes using peptide nucleic acid. Nucleic Acid Therapeutics 23(5): 363-7. IF=2.88. https://doi.org/10.1089/nat.2013.0426
27. J. Kuriakose, V. Hernandez-Gordillo, M. Nepal, A.a Brezden, V. Pozzi, M. N. Seleem, & J. Chmielewski. (2013). Targeting intracellular pathogenic bacteria with unnatural proline-rich peptides: coupling antibacterial activity with macrophage penetration. Angewandte Chemie International Edition 52(37):9664-7. IF=12.1. https://doi.org/10.1002/anie.201302693
26. M. N. Seleem, R. Vemulapalli, S. M. Boyle, G. G. Schurig & N. Sriranganathan. (2004). Improved expression vector for Brucella species. BioTechniques 37 (5): 740-744. IF= 2.75. https://doi.org/10.2144/04375BM05
25. A. Bandara, M. N. Seleem, C. Jordan, D. Lindsay, G. G. Schurig, & N. Sriranganathan. (2006). Brucella abortus strain RB51 can be used to express potentially protective antigens of Toxoplasma gondii. Journal of eukaryotic microbiology 53 (S1): S166–S168. IF= 2.91. https://doi.org/10.1111/j.1550-7408.2006.00218.x
24. A. Contreras, M. N. Seleem, S. M. Boyle, G. G. Schurig, N. Sriranganathan & A. Lopez-merino. (2006). Cloning, expression and characterization of immunogenic aminopeptidase N from Brucella melitensis. FEMS Immunology and Medical Microbiology 48 (2): 252-256. IF= 3.07. https://doi.org/10.1111/j.1574-695X.2006.00145.x
23. M. N. Seleem, M. Ali, S. M. Boyle, B. Mukhopadhyay, S. G. Witonsky, G. G. Schurig & N. Sriranganathan. (2006). Establishment of gene expression system in Ochrobactrum anthropi. Applied and Environmental Microbiology 72 (10): 6833-6836. IF= 3.95 . https://doi.org/10.1128/AEM.01446-06
22. M. N. Seleem, M. Ali, M. Abd Alazim, S. M. Boyle & N. Sriranganathan. (2007). High-level heterologous gene expression in Ochrobactrum anthropi using an A-rich UP element. Applied Microbiology and Biotechnology 73 (5):1123-1127. IF=3.81. https://doi.org/10.1007/s00253-006-0555-7
21. M. N. Seleem, M. Ali, M. Abd Alazim, S. M. Boyle & N. Sriranganathan. (2007). Enhanced expression, detection and purification of recombinant protein using tandem fusion tags RNA stem loop. Applied Microbiology and Biotechnology 75 (6):1385-1392. IF=3.81. https://doi.org/10.1007/s00253-007-0970-4
20. M. N. Seleem, S. M. Boyle & N. Sriranganathan. (2008). Brucella; a pathogen without classical virulence genes. Veterinary Microbiology 129 (1-2):1-14. IF=2.72. https://doi.org/10.1016/j.vetmic.2007.11.023
19. M. N. Seleem, P. Rajasekaran, M. Ali, S. M. Boyle & N. Sriranganathan. (2008). Simple Method for Transformation of Ochrobactrum anthropi. World Journal of Microbiology and Biotechnology 24 (10): 2111-2114. IF=2.10. https://doi.org/10.1007/s11274-008-9716-4
18. I. Sandal, M. N. Seleem, Elswaifi, N. Sriranganathan & T. Inzana. (2008). Construction of a high-efficiency shuttle vector for Histophilus somni. Journal of Microbiological Methods 74 (2-3):106-109. IF=2.09. https://doi.org/10.1016/j.mimet.2008.04.002
17. M. N. Seleem, M. Ali, S.M. Boyle & N. Sriranganathan. (2008). Vectors for enhanced gene expression and protein purification in Salmonella. Gene 421 (1-2): 95-98. IF=2.49. https://doi.org/10.1016/j.gene.2008.06.021
16. N. Pothayee, M. L. Vadala, A. Ranjan, N. Jain, M. N. Seleem, N. Sriranganathan & J. S. Riffle. (2008). Aminoglycoside-Ionopolymeric nanoplexes for targeting intracellular bacterial pathogens. Polymer Preprints 49 (2): 1036-1037. IF not determined. https://doi.org/10.1128/AAC.45.11.2977-2986.2001
15. M. N. Seleem, M. Ali, S. M. Boyle & N. Sriranganathan. (2008). Reporter genes for real-time in vivo monitoring of Ochrobactrum anthropi infection. FEMS Microbiology letters 286 (1):124-129. IF= 2.72. https://doi.org/10.1111/j.1574-6968.2008.01270.x
14. P. Rajasekaran, M. N. Seleem, A. Contreras, N. Sriranganathan & S. Boyle. (2008). A leucine auxotroph of Brucella abortus strain RB51 vaccine as an environmentally safe vector for plasmid maintenance and antigen expression. Applied and Environmental Microbiology 74 (22): 7051-7055. IF= 3.95 . https://doi.org/10.1128/AEM.01511-08
13. M. N. Seleem, N. Jain, H. Alqublan, R. Vemulapalli, S. M. Boyle, & N. Sriranganathan. (2008). Activity of native vs. synthetic promoters in Brucella. FEMS Microbiology Letters 288 (2): 211-215. IF= 2.72. https://doi.org/10.1111/j.1574-6968.2008.01358.x
12. P. Munusamy, M. N. Seleem, H. Alqublan, R. Tyler, N. Sriranganathan & G. Pickrell. (2009). Targeted drug delivery using silica xerogel systems to treat diseases due to intracellular pathogens. Material Science and Engineering C 29 (8): 2313-2318. IF=5.08. https://doi.org/10.1016/j.msec.2009.05.020
11. M. N. Seleem, Jain N., N. Pothayee., Ranjan A, J. Riffle & N. Sriranganathan. (2009). Targeting Brucella melitensis with polymeric nanoparticles containing streptomycin and doxycycline. FEMS Microbiology Letters 294 (1): 24-31. IF= 2.72. https://doi.org/10.1111/j.1574-6968.2009.01530.x
10. A. Ranjan, N. Pothayee, M. N. Seleem, R. Tyler, N. Sriranganathan, R. Kasimanickam, M. Makris & J. S. Riffle. (2009). Antibacterial efficacy of core-shell nanostructures encapsulating gentamicin against in vivointracellular Salmonella model. International Journal of Nanomedicine(4): 289-297. IF=4.37. https://doi.org/10.2147/IJN.S7137
9. P. Munusamy, M. N. Seleem, N. Sriranganathan & G. Pickrell. (2009). Synthesis and characterization of Magnetic Silica Xerogels for Targeting Intracellular Pathogens. Journal of Bionanoscience3 (1):16–21. IF not determined. https://doi.org/10.1166/jbns.2009.1004
8. A. Ranjan, N. Pothayee, M. N. Seleem, N. Sriranganathan, R. Kasimanickam, M. Makris & J. S. Riffle. (2009). In-vitro trafficking and efficacy of core-shell nanostructures for treating intracellular Salmonella. Antimicrobial Agents and Chemotherapy 53 (9): 3985-3988. IF= 4.45 . https://doi.org/10.1128/AAC.00009-09
7. D. Gillaspie, I. Perkins, K. Larsen, A. McCord, S. Pangonis, D. Sweger, M. N. Seleem, N. Sriranganathan, & B. Anderson. (2009). Plasmid based system for high-level gene expression and antisense gene knockdown in Bartonella. Applied and Environmental Microbiology75 (16):5434-5436. IF=3.95 . https://doi.org/10.1128/AEM.00949-09
6. M. N. Seleem, P. Munusamy, A. Ranjan, H. Alqublan, G. Pickrell & N. Sriranganathan. (2009). Silica-antibiotic hybrid nanoparticles for targeting intracellular pathogens. Antimicrobial Agents and Chemotherapy 53 (10):4270-4274. IF= 4.45 . https://doi.org/10.1128/AAC.00815-09
5. M. N. Seleem, S. M. Boyle & N. Sriranganathan. (2010). Brucellosis; A re-emerging zoonosis. Veterinary Microbiology 140 (3-4):392-398. IF=2.72 (Invited Review Article). The above manuscript is one of the most cited and most downloaded articles in Veterinary Microbiology since 2008. (1049 citations). https://doi.org/10.1016/j.vetmic.2009.06.021
4. H. Alqublan, M. N. Seleem, S. M. Boyle, & N. Sriranganathan. (2010). Tightly regulated expression system for Ochrobactrum. Current Microbiology 60 (4):242-247. IF = 1.37. https://doi.org/10.1007/s00284-009-9532-6
3. 16. A. Ranjan, N. Pothayee, M. N. Seleem, N. Jain, N. Sriranganathan, J. S. Riffle & R. Ksimanickam. (2010). Drug delivery using novel nanoplexes against a Salmonellamouse infection model. Journal of Nanoparticle Research 12 (3):905–914. IF = 2.27. https://doi.org/10.1007/s11051-009-9641-y
2. A. Ranjan, N. Pothayee, T. P. Vadala, M. N. Seleem, E. Restis, N. Sriranganathan, J. S. Riffle & R. Kasimanickam. (2010). Efficacy of amphiphilic Development of pluronic P85 based core-shell nanostructures encapsulating gentamicin against an in-vitro Salmonella and Listeria intracellular infection model. Antimicrobial Agents and Chemotherapy 54 (8):3524-3526. IF= 4.45 . https://doi.org/10.1128/AAC.01522-09
1. N. Surendran, K. Zimmerman , M. N. Seleem, N. Sriranganathan, S. M. Boyle, E. M. Hiltbold, H. Lawler, B. Heid & S. Witonsky. (2010). Ability of Brucella abortus rough vaccine strains to elicit DC and innate immunity in lung using a murine respiratory model. Vaccine 28 (43):7009-7015. IF= 3.48. https://doi.org/10.1016/j.vaccine.2010.08.039https://doi.org/10.1111/j.1574-6968.2008.01358.x
Patents
- Class II HMG-COA reductase inhibitors and methods of use. C. Stauffacher; M. Lipton; M. N. Seleem, T. Schmidt; N. Steussy, V. Rodwell. US Patent 9,073,832 (July 7, 2015)
- Antimicrobial substituted thiazoles and methods of use. M. S. Cushman, M. N. Seleem, AS Mayhoub. US Patent 9,353,072 (May 31, 2016)
- Class II HMG-COA reductase inhibitors and methods of use C. Stauffacher; M. Lipton; M. N. Seleem, T. Schmidt; N. Steussy, V. Rodwell. US Patent 9,604,925 (March 28, 2017)
- Antimicrobial substituted thiazoles and methods of use. M. S. Cushman, M. N. Seleem, AS Mayhoub. US Patent 9,801,861 (October 31, 2017)
- Lactones. (2018). M. Dai, M. N. Seleem, X. Yin. US Patent 10,087,190 (October 2, 2018)
- Lactones. (2018). M. Dai, M. N. Seleem, X. Yin. US Patent 10,138,252 (November 27, 2018)
- Aryl isonitriles as a new class of antimicrobial compounds. M. N. Seleem, M. Dai, D. C. Davis, H.T. Mohammad. US Patent 10,364,224 (July 2019)
- Phenylthiazoles and uses thereof. Mark Cushman, A. Mayhoub, M. N. Seleem. US Patent 10,414,759 (September 2019)
- Aryl isonitrile compounds as a new class of potent, broad-spectrum antifungal compounds. Kyei-Baffour; Kwaku, M. N. Seleem, Dai; Mingji, Mohammad; Haroon Taj. US Patent 10,449,174 (October 2019)
- Aryl isonitriles as a new class of antimicrobial compounds. M. N. Seleem, M. Dai, D. C. Davis, H.T. Mohammad. US Patent 11,091,437 (October 2019)
- Aryl isonitriles as a new class of antimicrobial compounds. M. N. Seleem, M. Dai, D. C. Davis, H.T. Mohammad. US Patent 11,098,014 (Nov 21, 2019)
- Repurposing non-antimicrobial drugs and clinical molecules to treat bacterial infections. M. N. Seleem. US Patent 10,301,664 (May 2019)
- Cleavable conjugates of antibiotics and an antibacterial cell-penetrating peptide. J. Chmielewski, M. N. Seleem. US Patent 10,265,408 (April 2019)
- Cleavable conjugates of antibiotics and an antibacterial cell-penetrating peptide. J. Chmielewski, M. N. Seleem. US Patent 10,688,192 (June 2020)
- Antibacterial cell-penetrating peptides. J. Chmielewski, M. N. Seleem. US Patent 10,875,895 (December 2020)
- Method and device for annihilation of methicillin-resistant Staphylococcus aureus. Cheng; Ji-Xin, M. N. Seleem, Dong; Pu-Ting, Hui; Jie. USA Patent 11,013,933 (May 2021)
- Aryl isonitriles as a new class of antimicrobial compounds. M. N. Seleem, M. Dai, D. C. Davis, H.T. Mohammad. US Patent 11,198,674 (December 2021)
- Safer, potent, and fast acting antimicrobial agents. PV Ramachandran, M Seleem.US Patent App. 17/220,974 (2021).
- Benzamide antibacterial agents. HO Sintim, M Seleem, C Opoku-Temeng, HT Mohammad, G Naclerio. US Patent App. 17/046,957. (2021).
- Diiodohydroxyquinoline for the treatment of clostridium difficile infection. M Seleem, N Abutaleb. US Patent App. 17/063,750. (2021).
- Method for the determination of antibiotic susceptibility through stimulated Raman metabolic imaging. Ji-Xin Cheng, M. N. Seleem, HONG Weili. US Patent 11,231,371 (January 2022)
- Carbonic anhydrase inhibitors and antibiotics against multidrug resistant bacteria. DP Flaherty, M Seleem, J Kaur, X Cao. US Patent App. 17/415,899 (2022).
- Carbonic anhydrase inhibitors for treatment of Neisseria gonorrhoeae infection DP Flaherty, M Seleem. US Patent App. 17/552,413 (2022).
- Method for the determination of antibiotic susceptibility through stimulated raman metabolic imaging. JX Cheng, M Seleem, H Weili. US Patent App. 17/548,483 (2023).
- 2023 US Patent# 11,731,964. Benzamide antibacterial agents.
- Diiodohydroxyquinoline for the treatment of clostridium difficile infection. M Seleem, N Abutaleb. US Patent App. 18/387,450 (2024).
2025
- We are proud to share that Ahmed Abouelkhair has been awarded Best Poster Presentation at the Virginia Tech Drug Discovery Center Symposium-VTCDD 2025 for outstanding work on drug discovery targeting Clostridioides difficile! 🎉 Congratulations on this well-deserved achievement.
- Congratulations to Somaia Abdelmegeed! Somaia won third place in the poster presentation at VCOM-VA Research Day! Well done, Somaia! We’re proud of you!
- Congratulations to Ahmed Abouelkhair! We’re excited to celebrate Ahmed, a Ph.D. candidate in our group, for receiving 1st place in the poster presentation at the CRWAD conference in Chicago, January 18–21.
- Congratulations to Ahmed Abouelkhair! We’re thrilled to celebrate Ahmed, a Ph. D. candidate in our group, for receiving the BMVS Travel Award to support his participation in the CRWAD conference in Chicago from January 18–21, where he will showcase his amazing work.
Congratulations, Ahmed, on this fantastic accomplishment!
-
Article Item
 Veterinary college promotes human, environmental, and animal health through One Health concept , article
Veterinary college promotes human, environmental, and animal health through One Health concept , articleOne Health is the overarching concept that human, animal, and environmental health are inextricably linked and that professionals within the three realms should work together toward research findings and clinical applications that can improve the health in all three areas.
-
Article Item
 Academic-private partnership aims to reduce toxic effects of deadly digestive bacteria , article
Academic-private partnership aims to reduce toxic effects of deadly digestive bacteria , articleMohamed Seleem and Nectagen Inc. have received a nearly $275,000 grant from the National Institute of Allergy and Infectious Diseases to study whether synthetic proteins developed by Nectagen can reduce the toxicity of the digestive bacteria commonly referred to as C. diff.
-
Article Item
 Center for One Health Research receives nearly $2 million from NIH to find new ways to combat gonorrhea , article
Center for One Health Research receives nearly $2 million from NIH to find new ways to combat gonorrhea , articleIn an era of “superbugs” such as strains of Neisseria gonorrhoeae resistant to antibiotics, the stakes are incomprehensibly high if new antibiotics and new ways of fighting disease-causing microbes are not found.
-
Article Item
 Mohamed Seleem named director of the Center for One Health Research , article
Mohamed Seleem named director of the Center for One Health Research , articleAs director, Seleem plans to develop bacteriology and infectious disease as the center's focus. Specializing and hiring more faculty in these fields will allow researchers to collaborate more and be more competitive when applying for funding.
- 🎉 Congratulations to Dr. Yehia Elgammal! 🎉
We are thrilled to announce that Yehia Elgammal has successfully defended his PhD! We couldn’t be prouder of his accomplishments and the impact he has made in his field. Yehia will continue his impactful work as a postdoctoral researcher in the esteemed lab of Dr. Del Poeta at Stony Brook University. Please join us in celebrating this incredible milestone and wishing Dr. Elgammal continued success in all his future endeavors! 🎓✨ - Congratulations to Ahmed Abouelkhair! We’re thrilled to share that Ahmed, a PhD candidate in our group, has been awarded the prestigious ACVM Graduate Student Travel Award! This recognition supports his attendance at the CRWAD conference in Chicago from January 18 - 21, where he will present his exciting work.Kudos to Ahmed Abouelkhair for this well-deserved achievement!
- Please join us for the final examination seminar of our PhD candidate, Yehia Elgammal. Title: "Discovery and development of novel antifungal agents for the treatment of Candida auris infections”. Yehia’s seminar will be held on Thursday, December 5th, at 1:00 PM in VMIA 220 Vet Med.
- Congratulations to Ahmed Abouelkhair for officially becoming a PhD candidate after successfully passing his preliminary exam! Fantastic achievement—keep up the amazing work!
- Huge congratulations to Nour Alkashif on officially becoming a PhD candidate after successfully passing his preliminary exam! Well done and keep up the great work!
- Meet Our New Team Member: Somaia, Master's Student at Dr. Seleem's Lab.
We are thrilled to introduce Somaia, who has joined Dr. Seleem's laboratory as a master's student. She specializes in developing antimicrobial peptides aimed at combating superbugs. Somaia brings a wealth of knowledge from her studies in veterinary medicine at Beni-Suef University, Egypt, where she graduated in 2014. Her journey includes significant experience as a Microbiologist at a human medical diagnostic laboratory, where she refined her skills in pathogen isolation, identification from clinical samples, and conducting antimicrobial susceptibility and serological tests. Somaia also completed a research internship at Purdue University in Indiana, where she worked on identifying molecular markers in dog mammary tumors as a predictive model for human breast cancer. We are thrilled to have Somaia join the Seleem team, and we eagerly anticipate the valuable contributions she will make. - Congratulations to Babatomiwa Kikiowo (Kiki) on completing his Master's thesis! A job well done, indeed. We are all incredibly proud of your hard work and dedication.
- Please join us for the Master of Science Seminar and Examination for Babatomiwa Kikiowo. https://bmvs.vetmed.vt.edu/events/student-defenses/kikiowo-babatomiwa.html
- Seleem lab members at the ASM Microbe 2024 Conference at Atalanta, Georgia, June 13th - June 17th!!
- Congratulations to our graduate students, Yehia Elgammal and Babatomiwa Kikiowo, for winning the BMVS Travel award! They will be representing us at the esteemed ASM Microbe conference in Atlanta from June 13th to 17th, showcasing their outstanding work. We are very pound of their achievements and anticipate their impactful contributions at this distinguished event!
- Congratulations, Ahmed Abouelkhair ! Ahmed's exceptional academic achievement and dedication have been recognized by Virginia Tech's chapter of Phi Kappa, ranking him in the top 10% of the PhD graduation class. This is a testament to his hard work, passion for learning, and commitment to excellence. We are incredibly proud of Ahmed's accomplishments and know that this is just the beginning of many more successes to come. Keep shining bright and inspiring others with your brilliance!
- We are delighted to welcome Prof. Christopher Lawrence as our new Group Manager. Prof. Lawrence brings a wealth of experience, expertise, and accomplishments from both industrial and academic backgrounds, making him an invaluable addition to our team. Dr. Lawrence’s primary research focus has been on host-pathogen interactions spanning genomics, immunology, biotechnology, and bioinformatics. With over 25 years of combined experience in academia and industry, Prof. Lawrence has showcased remarkable leadership skills and a consistent track record of success. Throughout his career, Prof. Lawrence has been honored with prestigious awards, including the NSF Plant Genome Young Investigator. Prof. Lawrence's contributions extend beyond accolades, with a significant impact reflected in his successful acquisition of over $20 million in extramural funding from NIH, NSF, USDA, and the Mayo Foundation. Additionally, he has delivered over 45 invited presentations in both national and international venues and has served on editorial boards, including as Associate Editor for Fungal Genetics and Biology and as Associate Editor for Frontiers in Fungal Biotechnology. We are thrilled to have Prof. Lawrence join the Seleem team, and we eagerly anticipate the valuable contributions he will make toward our shared goals.
- It is with great pleasure that we introduce Dr. Autumn Dove as the newest addition to Seleem's Lab as a Postdoctoral Researcher. Autumn received her PhD in Microbiology at the University of Florida. There, she worked with finding alternative therapies for antimicrobial-resistant infections caused by the ESKAPEE pathogens. Her primary efforts were using drug repurposing, metal nanoparticles, and host-targeting. She is looking forward to continuing to work with antimicrobial drug discovery! We are excited to welcome Dr. Dove to our team, and we look forward to the valuable insights and contributions she will bring to our ongoing research endeavors.
- We are thrilled to welcome Ammar Khan, the newest PhD student in the Seleem lab. Ammar brings a wealth of knowledge and experience to our team, holding a PharmD from Ziauddin University, Pakistan, and having recently completed his Master’s in Biomedical Innovation and Development at the Georgia Institute of Technology (Georgia Tech). Ammar’s diverse work experience in the pharmaceutical industry, including roles in commercialization and medical affairs teams, adds a unique perspective to our group. Beyond his professional accomplishments, Ammar enjoys cooking, board games, and playing soccer. We eagerly anticipate his valuable contributions to the group and are excited about the collaborative research endeavors ahead. Please join us in extending a warm welcome to Ammar and wishing him success in his journey with us.
- Dr. Ehab Salama joins Seleem's group as a Postdoc Research Associate
I am delighted to extend a warm welcome to our new postdoc Dr. Ehab Salama. Dr. Salama brings a wealth of expertise, holding a PharmD and a Master’s in Microbiology and Immunology from Al-Azhar University, Egypt. He recently earned his PhD from Virginia Tech, specializing in drug discovery, particularly antifungals. .He is an expert in drug discovery, particularly antifungals. We are thrilled to have him as a valued member of Seleem's group, and we look forward to the valuable contributions he will bring to our research endeavors. Please join me in welcoming Dr. Salama to our team!
- Congratulations, Dr. Ehab Salama, on your remarkable PhD thesis defense and completing your PhD! You have truly earned this well-deserved achievement.
- Congratulations to Nicholas Burns (Nic) who finished his Master's thesis with us! A job well done.
- Dr. Seleem was featured in the news as the Director of the Center for One Health Research, showcasing the center's activities and accomplishments.
https://news.vt.edu/articles/2023/12/vetmed-one-health.html?utm_source=cmpgn_news&utm_medium=email&utm_campaign=vtUnirelNewsDailyCMP_news-fs-120423 - Please join us for the PhD defense of Ehab Salama https://emma-assets.s3.amazonaws.com/6lc/92dfa9e6b0d85d811dbce16537aa58c3/Ehab_Salama_PhD.pdf
- Please join us for the Master of Science Seminar and Examination for Nicolas D. Bruns. https://emma-assets.s3.amazonaws.com/6lc/6762d0de58ea9355f3ec747170a1eed7/Nic_Burns_MS.pdf
- Congratulations to Yehia Elgammal, who has now become a PhD candidate, for successfully passing his preliminary exam. Great Job!
- Thrilled to share a proud moment as a PI — Dr. Shankar Thangamani, my first PhD student, has achieved a significant milestone with his first R01 grant! Congratulations on this remarkable achievement!
- Our lab has been featured in multiple news articles, highlighting our recent achievements and collaborations with Nectagen:
https://news.vt.edu/articles/2023/08/vetmed-seleem-nectagen-grant.html
https://www.the-microbiologist.com/news/academic-private-partnership-aims-to-reduce-toxic-effects-of-deadly-c-diff/1430.article
https://www.newswise.com/articles/academic-private-partnership-aims-to-reduce-toxic-effects-of-deadly-digestive-bacteria
https://www.digitaljournal.com/tech-science/new-partnership-seeks-to-reduce-toxic-effects-of-pathogenic-digestive-bacteria/article
https://www.eurekalert.org/news-releases/998137 - Brice Stolz joins Seleem's group
I am delighted to extend a warm welcome to our new PhD student, Brice Stolz. Brice holds a Bachelor of Science in Cellular, Molecular, and Physiological Biology, with a minor in Communication Studies, earned from Christopher Newport University in 2023. During his undergraduate studies, he demonstrated a keen interest in anti-cancer drug design through his research efforts. Aside from his academic achievements, Brice is also an Eagle Scout, embracing various adventurous activities such as camping, hiking, backpacking, and cycling. We are thrilled to have him as a valued member of Seleem's group, and we look forward to the valuable contributions he will bring to our research endeavors. Please join me in welcoming Brice to our team! - Our lab was featured in several news articles
Our lab was featured in several news articles detailing the potential use of atazanavir, an HIV protease inhibitor drug, as a new avenue to improving the effectiveness of existing antifungals for those with a Candida auris infection. The study was led by two very talented scientists from our group, Yehia Elgammal and Ehab Salama.
https://news.vt.edu/articles/2023/05/vetmed-candida-auris-research.html
https://www.news-medical.net/news/20230525/Antiviral-drugs-may-be-a-new-treatment-strategy-in-the-fight-against-Candida-auris.aspx
https://www.laboratoryequipment.com/597253-HIV-Drug-May-Help-Fight-Deadly-Candida-Auris-Pathogen/
https://microbionews.com/2023/05/26/antiviral-drugs-may-be-a-new-treatment-strategy-in-the-fight-against-candida-auris/
https://www.eurekalert.org/news-releases/990462 - Cindy's Farewell and Graduation celebration!
Congratulations Hsin-Wen Liang (Cindy), for accepting the offer of admission to her top choice Ph.D. program after getting multiple Ph.D. offers from prestigious programs. Congratulations Cindy!!! - Congratulations to Cindy for successfully defending her Master's thesis!
Congratulations to Hsin-Wen Liang (Cindy) who finished her Master's thesis with us! A job well done. - The Master of Science Seminar and Examination of Hsin-Wen Liang VMIA 220 and Zoom: 88323892950, Passcode: Cindy
- Congratulations Cindy for winning the best poster presentation!
Congratulations Hsin-Wen Liang (Cindy) for winning the best poster presentation award at 39th Annual GPSS Research Symposium 2023 at VT. Well done Cindy! - Congratulations Ehab for winning the best poster presentation!
Congratulations Ehab Salama for winning the best poster presentation award at 39th Annual GPSS Research Symposium 2023 at VT. Well done Ehab! - Congratulations Cindy for winning the best oral presentation!
Congratulations to Hsin-Wen Liang (Cindy) who just won the best oral presentation Award at the 32nd Annual Graduate Research Symposium at Virginia Tech. Well done Cindy! - Please join us in welcoming our new Research Scientist Dr. Mohamed F. Mohamed. Mohamed
Dr. Mohamed F. Mohamed. Mohamed finished his PhD at the College of Veterinary Medicine, Purdue University at Seleem’s lab. Then, he joined Rush University Medical Center at Chicago as a postdoctoral fellow under the direction of Professor Sasha Shafikhani working on bacterial pathogenesis and innate and translational immunity. He is an exceptional scientist and bacteriologist with immense experience in antimicrobial drug discovery. He has an extensive publication record including Nature communications, eLife, JACS, and Journal of Controlled Release. We are excited to welcome Mohamed again back to our team !! - Congratulations to Dr. Haroon Mohammad who made it to the Stanford University’s top 2% of scientists list
Congratulations to Dr. Haroon Mohammad, our former PhD student and postdoc who made it to the Stanford University’s top 2% of scientists list that includes the most-cited, influential, and distinguished scientists around the world. - Please join us in welcoming our new PhD student Abdallah Abdelsattar (Adam)
Adam obtained his baccalaureate in Biomedical Sciences (BMS) with a concentration in Drug Design and minor in Nanoscience in 2018 from the University of Science and Technology, Zewail City, Egypt. Then, he finished his Master degree from the same University at El-Shibiny's lab working on bacteriophage and nanoparticles. Adam started his PhD in Seleem's lab with a focus on novel approaches targeting multidrug resistant-pathogens.
- Rusha's Farewell and Graduation celebration!
We’ll miss you dearly but cannot wait to see your work in print!! - Congratulations to Ehab Salama on passing his prelim exams (written and oral) with flying colors !!
- Congratulations Rusha on the impressive defense of your PhD thesis!!
- Congratulations to our PhD candidate Rusha Pal on her Postdoctoral position at Harvard University!
Rusha accepted a position as a Postdoctoral Fellow at Beth Israel Deaconess Medical Center which is affiliated to the prestigious Harvard Medical School, Harvard University. She will be working in Dr. Ciaron Kelly and Dr. Xinhua Chen’s lab studying the epithelial and vascular barrier function in gut infections, particularly Clostridium difficile infection. Dr. Kelly and Dr. Chen established the Clostridium difficile animal model that is currently being used all over the world. Their group, one of the leading groups in the world, is working on gut microbiome and C. difficile infections. Congratulations, Rusha! Please Join us in congratulating Rusha! - Our lab was featured in several news articles:
https://cardinalnews.org/2022/11/10/virginia-tech-center-receives-2-million-nih-grant-for-std-research-more/
https://www.eurekalert.org/news-releases/969173
https://vtx.vt.edu/articles/2022/10/vetmed-gonorrhea.html
https://localtoday.news/va/virginia-tech-center-receives-nearly-2-million-nih-grant-for-std-research-more-48447.html - Congratulations to Rusha Pal for winning Best Poster Award at the 2022 CeZAP Infectious Diseases Symposium at Virginia Tech
Congratulations to Rusha Pal for winning Best Poster Award at the 2022 CeZAP Infectious Diseases Symposium at Virginia Tech, Blacksburg, VA. October 2022. - Celebration of NIH R01 Award at Cabo Fish Taco
Seleem Lab celebrating NIH R01 Award at Cabo Fish Taco. Nice food, amazing group!
- Congratulations to Hassan on his new post-doctoral position at University of Washington!
Dr. Hassan Eldesouky accepted a position as a Postdoctoral Fellow in the prestigious Department of Microbiology, School of Medicine, University of Washington. He will be working in Dr. David Sherman’s lab studying the molecular genetics, systems biology, pathogenesis, and drug discovery of M. tuberculosis. Dr. Sherman played a lead role in the discovery and early development of the anti-TB agent pretomanid. In addition, his laboratory defined the mutation responsible for attenuation of the world’s most widely used vaccine, BCG. Congratulations, Hassan! - Congratulations to our Ph.D. student Rusha Pal on successfully passing her prelim exams !!
- Congratulations to our lab member Dr. Nader Abutaleb for successfully defending his Ph.D. thesis
Congratulations to Dr. Nader Abutaleb on successfully defending his Ph.D. thesis with a record number of publications (41) !! We wish him success in all his future endeavors !! - Our Work was featured on Contagion Live
https://www.contagionlive.com/view/repurposing-a-veterinary-antiprotozoal-to-replace-metronidazole-for-c-difficile
Current Funding
We are thankful for current financial support from:
- National Institutes of Health (NIH)
- National Institute of Allergy and Infectious Diseases (NIAID)
- National Science Foundation (NSF)
- National Academy of Sciences (NAS)
- Indiana Clinical and Translational Sciences Institute (CTSI)
- Egyptian Cultural and Educational Bureau (ECEB)
- Tyler J. and Frances F. Young Endowment
Previous Funding
We are thankful for previous financial support that came from:
- National Institutes of Health (NIH)
- National Institute of Allergy and Infectious Diseases (NIAID)
- National Science Foundation (NSF)
- National Institute of Food and Agriculture (NIFA)
- Showalter Research Trust
- Egyptian Cultural and Educational Bureau (ECEB)
- Purdue Research Foundation
- The Office of Agricultural Research at Purdue University
- AgSEED- Agricultural Research and Extension Leading to Economic Development in Indiana Agriculture and Rural Communities.
- Office of the Executive Vice President for Research and Partnerships- Purdue University
- Purdue Institute for Drug Discovery - Purdue University
- Purdue Institute of Inflammation, Immunology and Infectious Disease- Purdue University














