Luo Laboratory

About the Luo Lab
Our research interests are autoimmunity, host-microbe interactions, neonatal immunity, and nutritional immunology. There are 3 main directions of research in our laboratory. The first is to determine the role of gut microbiota in the pathogenesis of lupus. We have found that a leaky gut drives autoimmunity, and we are currently delineating the underlying mechanisms. The second direction is to reveal novel mechanisms of microbiota-mediated regulation of neonatal immune development, and how immunological imprints during the neonatal stage would impact the development of autoimmunity later in life. The third direction is focused on the role of vitamin A in the pathogenesis of lupus.
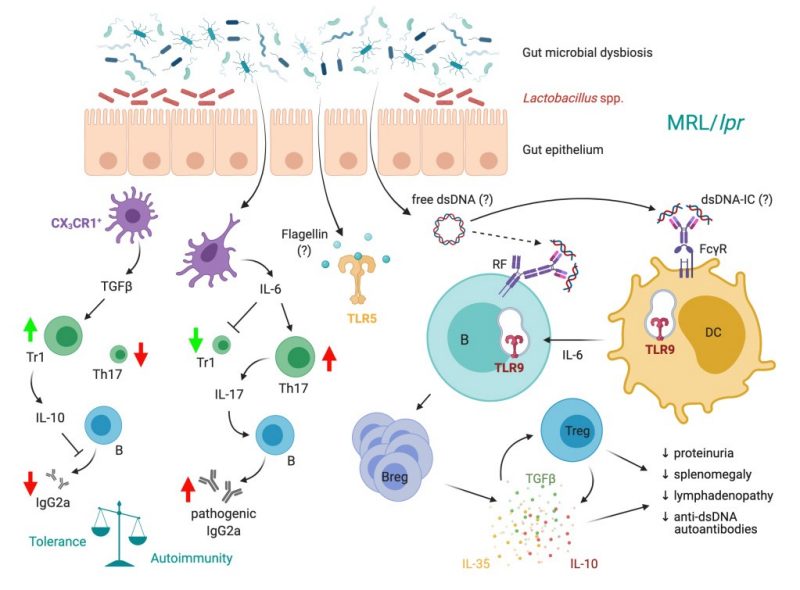
Systemic lupus erythematosus (SLE) is a complex autoimmune disorder with no known cure. Increasing evidence in recent years suggest that microbiota—largely commensal bacteria living in our gut—and the immune system interact to maintain tissue homeostasis, but the involvement of this interaction in the pathogenesis of SLE remains unclear. Our research team was the first to describe changes of gut microbiota in lupus mice vs. healthy controls, with lupus mice having a decrease of Lactobacillaceae and an increase of Lachnospiraceae. Based on this observation, we administered a mixture of 5 strains of Lactobacillus spp. into lupus mice via oral gavage, which significantly attenuated the disease. This follow-up study, which was done using MRL/lpr mice, along with two recent studies using additional models of murine lupus, established gut microbiota as a cause, instead of effect, of SLE pathogenesis. Our ultimate goal is to mechanistically define the role of gut microbiota in the pathogenesis of SLE.
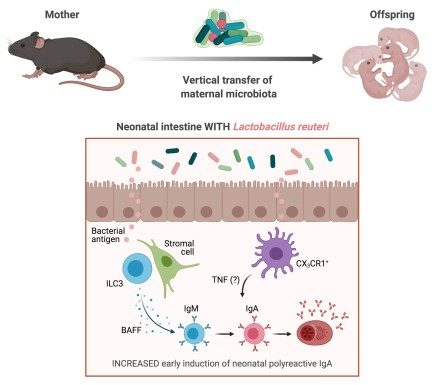
Infants are prone to enteric infections due to an underdeveloped immune system. Maternal microbiota, through shaping the neonatal microbiota, helps establish a strong immune system in infants. We and others have observed the phenomenon of enhanced early neonatal IgA production in pre-weaning immunocompetent mice nursed on immunodeficient dams. Here, we show that this enhancement of IgA in neonates results from maternally derived microbiota. In addition, we have found that the neonatal IgA production can be induced by Lactobacillus reuteri, which is enriched in the milk of immunodeficient dams. Moreover, we show that while the production of neonatal IgA is dependent on neonatal T cells, the immunodeficient maternal microbiota-mediated enhancement of neonatal IgA has a T cell-independent component. Indeed, this enhancement may be dependent on type 3 innate lymphoid cells in the neonatal small intestinal lamina propria. Interestingly, maternal microbiota-induced neonatal IgA does not cross-react with common enteric pathogens. Future investigations will determine the functional consequences of having this extra IgA.
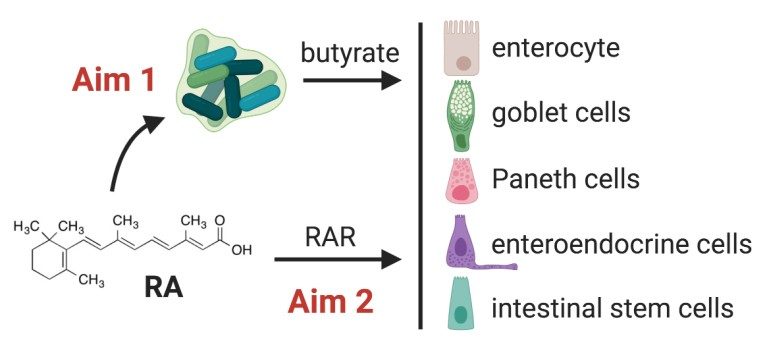
Vitamin A plays important roles in a wide range of biological processes. Among these is immune modulation, where vitamin A is required for the development and maintenance of a functional immune system. Most effects of vitamin A are exerted by its active metabolite, retinoic acid (RA), which through interaction with its receptors (RAR) controls the transcription of RA-responsive genes. In the past 10 years, much interest has been focused on the role of RA on the intestinal mucosa. The current literature emphasize that RA maintains gut homeostasis by regulating gut-homing of many immune cell types. However, the effect of RA on intestinal epithelial cells (IECs) has been less well studied. The function of IECs as regulators of gut barrier function mandates a more complete analysis of these cells. Leaky gut is present in numerous autoimmune diseases, and may exacerbate the autoimmune state. RA may reverse a leaky gut either (or both) by: 1) direct regulation of the intestinal epithelium, and/or 2) modulation of gut microbiota. We hypothesize that RA affects multiple types of IECs and enhances intestinal epithelial homeostasis both directly and through increasing butyrate-producing commensal bacteria in the gut.
Principal Investigator
Xin M. Luo, PhD
Assistant Department Head for Graduate and Postgraduate Studies
Professor, Immunology
xinluo@vt.edu
Current Lab Members

Ran Lu, PhD
Senior Research Associate

Noah Oakland
MD/PhD candidate

Rana Estaleen
PhD candidate

Caitlin Armstrong
PhD student

Tian Xu
PhD student

Pavly Amin
Undergraduate student (2023-present)

Kian Mabudian
Undergraduate student (2022-present)

Aida Shakeri
Undergraduate student (2022-present)
Alumni
- Xiaofeng Liao, PhD (Scientist, biotech industry)
- Qinghui Mu, PhD (Postdoc, Stanford)
- Jingjing Ren, MD, PhD (Postdoc, Yale)
- Michael Edwards, DVM, PhD (Clinical Assistant Professor, VMCVM)
- Brianna Swartwout, PhD (Teacher, Teach for America)
- Jiangdi Mao (PhD student, Zhejiang University)
- Jing Zhu, PhD (Scientist, biotech industry)
- Leila Abdelhamid, PhD (Postdoc, FDA)
- Fernando Garcia (PhD student, Brown University)
- Xavier Cabana-Puig, PhD (Scientist, biotech industry)
- Michael Appiah, PhD (Postdoc, Mount Sinai)
- Razan Alajoleen, PhD (Scientist, Virginia Tech)
- Maksimiano Rodríguez, Exchange student (2023)
- Fernando Garcia, PREP student (2021 – 2022)
- Noah Oakland, MD student (2021 – 2022)
- Amy Lin, DVM student (2021)
- Nathaniel Cheung, High school student (2021)
- Constanza Sangmeister, Exchange student (2019)
- Grace Lee, MD student (2018 – 2020)
- Meeta Prakash, MD student (2018 – 2020)
- Jiangdi Mao, MS student (2019)
- Sabrina Callaway, DVM student (2018)
- Caleb Whitfield, DVM student (2017)
- Vincent Tavella, DVM student (2016)
- John Gil, DVM student (2013)
- Joshua Sparks, MD student (2012 – 2014)
- Brent Gordon, DVM student (2012)
- Tian Xu, Undergraduate student (2022 – 2023)
- James Testerman, Undergraduate student (2020 – 2023)
- Anna Christovich, Undegraduate student (2020 – 2022)
- Erica Giles, Undegraduate student (2019 – 2020)
- Kate Hardin, Undergraduate student (2018 – 2020)
- Jacob Bond, Undergraduate student (2018 – 2019)
- Mellanee Gilkerson, Undergraduate student (2016)
- Jillian Kazmierczak, Undergraduate student (2016)
- Reilly Scott, Undergraduate student (2016)
- Jay Kirby, Undergraduate student (2015 – 2018)
- Tharshikha Pirapakaran, Undergraduate student (2015 – 2016)
- Alec Reihl, Undergraduate student (2015 – 2016)
- David Branson, Undergraduate student (2015 – 2016)
- Jennifer Zumbo, Undergraduate student (2015)
2024
- Tang W, Wei Y, Ni Z, Hou K, Luo XM, Wang H (2024) IgA-mediated control of host-microbial interaction during weaning reaction influences gut inflammation. Gut Microbes 16(1):2323220.
- Jin Q, Feng Y, Cabana-Puig X, Chau TN, Difulvio R, Yu D, Hu A, Li S, Luo XM, Ogejo J, Lin F, Huang H (2024) Combined dilute alkali and milling process enhances the functionality and gut microbiota fermentability of insoluble corn fiber. Food Chemistry 446:138815.
- Gutierrez F, Murphy QM, Swartwout BK, Read AR, Edwards MR, Abdelhamid L, Cabana-Puig X, Testerman JC, Xu T, Lu R, Amin P, Cecere TE, Reilly CM, Oestreich KJ, Ciupe SM, Luo XM (2024) TCDD and CH223191 alter T cell balance but fail to induce anti-inflammatory response in adult lupus mice. ImmunoHorizons 8(2):172-181.
- Dai J, Mao J, Wei Y, Hou K, Luo XM, Wang H (2024) Soybean agglutinin alters the gut microbiota and promotes inflammation in lupus-prone MRL/lpr mice. Journal of Nutrition 154(3):1039-49
- Estaleen R, Reilly CM, Luo XM (2024) A double-edged sword: Interactions of CX3CR1/CX3CL1 and gut microbiota in systemic lupus erythematosus. Frontiers in Immunology 14:1330500.
2023
- Daamen AR, Alajoleen RM, Grammer AC, Luo XM and Lipsky PE (2023) Single-cell RNA sequencing analysis reveals the heterogeneity of IL-10 producing regulatory B cells in lupus-prone mice. Frontiers in Immunology 14:1282770.
- Cabana-Puig X, Lu R, Geng S, Michaelis JS, Oakes V, Armstrong C, Testerman JC, Liao X, Alajoleen R, Appiah M, Zhang Y, Reilly CM, Li L, Luo XM (2023) CX3CR1 modulates SLE-associated glomerulonephritis and cardiovascular disease in MRL/lpr mice. Inflammation Research 72(5):1083-1097.
- Abdelhamid L, Mao J, Cabana-Puig X, Zhu J, Swartwout BK, Edwards MR, Testerman J, Michaelis JS, Allen IC, Ahmed SA, Luo XM (2023) Nlrp12 deficiency alters gut microbiota and ameliorates Faslpr -mediated systemic autoimmunity in male mice. Frontiers in Immunology 14:1120958.
- Zhu J, Naughton S, Bowman M, LeRoith T, Luo XM, Leeth C (2023) Maternal antibody repertoire restriction modulates the development of lupus-like disease in BXSB offspring. International Immunology 35(2):95-104.
- Abdelhamid L, Alajoleen R, Kingsmore K, Cabana-Puig X, Lu R, Zhu J, Testerman J, Li Y, Ross AC, Cecere TE, Reilly CM, Grammer A, Lipsky PE, Luo XM (2023) Hypovitaminosis A drives the progression of tubulointerstitial lupus nephritis through potentiating pre-disease cellular autoreactivity. ImmunoHorizons 7(1):17-29.
2022
- Cabana-Puig X, Mu Q, Lu R, Swartwout B, Abdelhamid L, Zhu J, Prakash M, Cecere TE, Wang Z, Callaway S, Sun S, Reilly CM, Ahmed SA, Luo XM (2022) Lactobacillus spp. act in synergy to attenuate splenomegaly and lymphadenopathy in lupus-prone MRL/lpr mice. Frontiers in Immunology 13:923754.
- Cabana-Puig X, Reilly CM, Luo XM (2022) Analysis of fecal microbiota dynamics in lupus-prone mice using a simple, cost-effective DNA isolation method. Journal of Visualized Experiments (183) e63623, doi: 10.3791/63623.
- Cabana-Puig X, Luo XM (2022) Analyses of proteinuria, renal infiltration of leukocytes, and renal deposition of proteins in lupus-prone MRL/lpr mice. Journal of Visualized Experiments (184) e63506, doi: 10.3791/63506.
- Christovich A, Luo XM (2022) Gut microbiota, leaky gut, and autoimmune diseases. Frontiers in Immunology 12:946248.
- Cabana-Puig X, Bond JM, Wang Z, Dai R, Lu R, Lin A, Oakes V, Rizzo A, Swartwout BK, Abdelhamid L, Mao J, Prakash M, Sangmeister C, Cheung N, Cowan C, Reilly CM, Sun S, Ahmed SA, Luo XM (2022) Phenotypic drift in lupus-prone MRL/lpr mice: Potential roles of microRNAs and gut microbiota. ImmunoHorizons, 6(1):36-46.
- Abdelhamid L, Luo XM (2022) Diet and hygiene in modulating autoimmunity during the pandemic era. Frontiers in Immunology, 12:749774.
2021
- Mu Q, Swartwout BK, Edwards MR, Zhu J, Lee G, Eden K, Cabana-Puig X, McDaniel DK, Mao J, Abdelhamid L, Brock RM, Allen IC, Reilly CM, Luo XM (2021) Regulation of neonatal IgA production by the maternal microbiota. Proceedings of the National Academy of Sciences 118 (9): e2015691118.
2020
- Mu Q, Edwards MR, Swartwout BK, Cabana-Puig X, Mao J, Zhu J, Grieco J, Cecere TE, Prakash M, Reilly CM, Puglisi C, Bachali P, Grammer AC, Lipsky PE, Luo XM (2020) Gut microbiota and bacterial DNA suppress autoimmunity by stimulating regulatory B cells in a murine model of lupus. Frontiers in Immunology 11:593353.
- Abdelhamid L, Cabana-Puig X, Mu Q, Moarefian M, Swartwout B, Eden K, Das P, Seguin RP, Xu L, Lowen S, Lavani M, Hrubec TC, Jones CN, Luo XM (2020) Quaternary ammonium compound disinfectants reduce lupus-associated splenomegaly by targeting neutrophil migration and T-cell fate. Frontiers in Immunology 11:575179.
- Abdelhamid L, Cabana-Puig X, Swartwout B, Lee J, Li S, Sun S, Li Y, Ross AC, Cecere TE, LeRoith T, Werre SR, Wang H, Reilly CM, Luo XM (2020) Retinoic acid exerts disease stage-dependent effects on pristane-induced lupus. Frontiers in Immunology 11:408.
- Menarim BC, Gillis KH, Oliver A, Mason C, Werre SR, Luo XM, Byron CR, Kalbfleisch TS, MacLeod JN, Dahlgren LA (2020) Inflamed synovial fluid induces a homeostatic response in bone marrow mononuclear cells in vitro: Implications for joint therapy. FASEB Journal 34(3):4430.
2019 or earlier
- Mu Q, Cabana-Puig X, Mao J, Swartwout B, Abdelhamid L, Cecere TE, Wang H, Reilly CM, Luo XM (2019) Pregnancy and lactation interfere with the response of autoimmunity to modulation of gut microbiota. Microbiome 7(1):105.
- Ren J, Catalina MD, Eden K, Liao X, Read KA, Luo XM, McMillan RP, Hulver MW, Jarpe M, Bachali P, Grammer AC, Lipsky PE, Reilly CM (2019) Selective histone deacetylase 6 inhibition normalizes B cell activation and germinal center formation in a model of systemic lupus erythematosus. Frontiers in Immunology 10:2512.
- Menarim BC, Gillis KH, Oliver A, Mason C, Ngo Y, Werre SR, Barrett SH, Luo XM, Byron CR, Dahlgren LA (2019) Autologous bone marrow mononuclear cells modulate joint homeostasis in an equine in vivo model of synovitis. FASEB Journal 33(12):14337.
- Swartwout B, Luo XM (2018) Implications of probiotics on the maternal-neonatal interface: gut microbiome, immunomodulation, and autoimmunity. Frontiers in Immunology 9:2840.
- Abdelhamid L, Luo XM (2018) Retinoid acid, leaky gut, and autoimmune diseases. Nutrients 10(8).
- Mu Q, Tavella VJ, Luo XM (2018) Role of Lactobacillus reuteri in human health and disease. Frontiers in Microbiology 9:757.
- Luo XM, Edwards MR, Mu Q, Yu Y, Vieson MD, Reilly CM, Ahmed SA, Bankole AA (2018) Gut microbiota in human systemic lupus erythematosus and a mouse model of lupus. Applied and Environmental Microbiology 84(4). pii: e02288-17.
- Reinoso Webb CR, den Bakker H, Kovoziev I, Jones-Hall Y, Kottapalli KR, Ostanin D, Furr KL, Mu Q, Luo XM, Grisham M. (2018) Differential susceptibility to T cell-induced colitis in mice: role of the intestinal microbiota. Inflammatory Bowel Diseases 24(2):361-379.
- Mu Q, Zhang H, Liao X, Lin K, Liu H, Edwards MR, Ahmed SA, Yuan R, Li L, Cecere TE, Branson DB, Kirby JL, Goswami P, Leeth CM, Read KA, Oestreich KJ, Vieson MD, Reilly CM, Luo XM. (2017) Control of lupus nephritis by changes of gut microbiota. Microbiome 5(1): 73.
- Mu Q, Tavella VJ, Kirby JL, Cecere TE, Chung M, Lee J, Li S, Ahmed SA, Eden K, Allen IC, Reilly CM, Luo XM (2017) Antibiotics ameliorate lupus-like symptoms in mice. Scientific Reports 7:13675.
- Mu Q, Kirby JL, Reilly CM, Luo XM. (2017) Leaky gut as a danger signal for autoimmune diseases. Frontiers in Immunology doi: 10.3389/fimmu.2017.00598.
- Liao X, Ren J, Reihl A, Pirapakaran T, Sreekumar B, Cecere TE, Reilly CM, Luo XM. (2017) Renal-infiltrating CD11c+ cells are pathogenic in murine lupus nephritis through promoting CD4+ T cell responses. Clinical and Experimental Immunology Jul 19. doi: 10.1111/cei.13017.
- Ren J, Liao X, Vieson MD, Chen M, Scott R, Kazmierczak J, Luo XM, Reilly CM. (2017) Selective HDAC6 inhibition decreases early stage of lupus nephritis by downregulating both innate and adaptive immune responses. Clinical and Experimental Immunology Sep 6. doi: 10.1111/cei.13046.
- Edwards MR, Dai R, Heid B, Cecere TE, Khan D, Mu Q, Cowan C, Luo XM, Ahmed SA. (2017) Commercial rodent diets differentially regulate autoimmune glomerulonephritis, epigenetics, and microbiota in MRL/lpr mice. International Immunology Jun 15. doi: 10.1093/intimm/dxx033.
- Vieson MD, Gojmerac AM, Khan D, Dai R, van Duzer JH, Mazitschek R, Caudell DL, Liao X, Luo XM, Reilly CM. (2017) Treatment with a selective histone deacetylase 6 inhibitor decreases lupus nephritis in NZB/W mice. Journal Histology and Histopathology Feb 28:11885. doi: 10.14670/HH-11-885.
- Theus MH, Sparks JB, Liao X, Ren J, Luo XM. (2017) All-trans-retinoic acid augments the histopathological outcome of neuroinflammation and neurodegeneration in lupus-prone MRL/lpr mice. Journal of Histochemistry and Cytochemistry 65(2): 69-81.
- Luo XM, Edwards MR, Reilly CM, Mu Q, Ahmed SA. (2017) Diet and microbes in the pathogenesis of lupus. Book chapter in Lupus. ISBN 978-953-51-5383-2.
- Vieson MD, Luo XM, Li S, Gojmerac AM, Castaneda A, Reilly CM. (2016) Selective HDAC6 inhibition corrects aberrant B cell development in the bone marrow of NZB/W F1 mice. Cellular & Molecular Medicine 2(3): 11.
- Liao X, Makris MR, Luo XM. (2016) Fluorescence-activated cell sorting for purification of plasmacytoid dendritic cells from the mouse bone marrow. Journal of Visualized Experiments 117. doi: 10.3791/54641.
- Liao X, Pirapakaran T, Luo XM. (2016) Chemokines and chemokine receptors in the development of lupus nephritis. Mediators of Inflammation 2016: 6012715.
- Liao X, Reihl AM, Luo XM. (2016) Breakdown of immune tolerance in systemic lupus erythematosus by dendritic cells. Journal of Immunology Research 5(suppl 39): 1-15.
- Regna NL, Vieson MD, Chafin CB, Puthiyaveetil AG, Hammond SE, Luo XM, Caudell DL, Jarpe MB, Reilly CM. (2016) Specific HDAC6 inhibition by ACY-738 reduces SLE pathogenesis in NZB/W mice. Clinical Immunology 162: 58-73.
- Mu Q, Zhang H, Luo XM (2015) SLE: Another autoimmune disorder influenced by microbes and diet? Frontiers in Immunology doi: 10.3389/fimmu.2015.00608.
- Liao X, Li S, Settlage RE, Sun S, Ren J, Reihl AM, Zhang H, Karyala SV, Reilly CM, Ahmed SA, Luo XM. (2015) Cutting edge: Plasmacytoid dendritic cells in late-stage lupus mice defective in producing IFN-α. Journal of Immunology 195: 4578-4582.
- Regna NL, Vieson MD, Gojmerac AM, Luo XM, Caudell DL, Reilly CM. (2015) HDAC expression and activity is upregulated in diseased lupus-prone mice. International Immunopharmacology S1567-5769(15)30139-9.
- Zhang H, Luo XM. (2015) Control of commensal microbiota by the adaptive immune system. Gut Microbes 6(2): 156-60.
- Liao X, Ren J, Wei CH, Ross AC, Cecere TE, Jortner BS, Ahmed SA, Luo XM. (2015) Paradoxical effects of all-trans-retinoic acid on lupus-like disease in the MRL/lpr mouse model. PLoS One 10(3): e0118176.
- Zhang H, Sparks JB, Karyala SV, Settlage R, Luo XM. (2014) Host adaptive immunity alters gut microbiota. ISME Journal 9(3): 770-81.
- Zhang H, Liao X, Sparks JB, Luo XM. (2014) Dynamics of gut microbiota in autoimmune lupus. Applied Environmental Microbiology 80(24): 7551-60.
- Luo XM, Lei MYY. (2012) Recombination activating gene-2 regulates CpG-mediated interferon-alpha production in mouse bone marrow-derived plasmacytoid dendritic cells. PLoS One 7(10): e47952.
- Yusuf D, Luo XM, et al. (2012) The transcription factor encyclopedia. Genome Biology 13(3): R24.
- Luo XM, Lei MYY, Feidi RA, West AP, Balazs AB, Bjorkman PJ, Yang L, Baltimore D. (2010) Dimeric 2G12 as a potent protection against HIV-1. PLoS Pathogens 6(12): e1001225.
- Luo XM, Maarschalk E, O’Connell RM, Wang P, Yang L, Baltimore D. (2009) Engineering human hematopoietic stem/progenitor cells to produce a broadly neutralizing anti-HIV antibody after in vitro maturation to human B lymphocytes. Blood 113(7): 1422-1431.
- Luo XM, Ross AC. (2006) Retinoic acid exerts dual regulatory actions on the expression and nuclear localization of interferon regulatory factor-1. Experimental Biology and Medicine 231(5): 619-631.
- Luo XM, Ross AC. (2005) Physiological and receptor-selective retinoids modulate interferon-g signaling by increasing the expression, nuclear localization, and functional activity of interferon regulatory factor-1. Journal of Biological Chemistry 280(43): 36228-36236.
- Luo XM, Fosmire GJ, Leach RM Jr. (2002) Chicken keel cartilage as a source of chondroitin sulfate. Poultry Science 81(7): 1086-1089.
- November 2023: Razan Alajoleen successfully defended her PhD dissertation! Congratulations Razan!
- June 2023: Xin M. Luo was promoted to full professor. She went on sabbatical for two months as a visiting professor at Department of Microbiology and Immunology in University of Otago, New Zealand.
- April 2023: The Luo Laboratory has been awarded a DoD Lupus Idea Award.
- October 2022: The Luo Laboratory has been awarded an iTHRIV pilot grant in collaboration with Carilion Clinic to study infant stool microbiome.
- August 2022: Xin M. Luo was interviewed by Researcher. Listen to the interview here. She was also interviewed by MIT Technology Review about her work at Caltech. Read the article here.
- July 2022: Xavier Cabana-Puig successfully defended his PhD dissertation! Congratulations Xavier!
- May 2022: Noah Oakland decided to continue in the lab as a MD/PhD student!
- February 2022: The Luo Lab celebrates 10-year anniversary!
- November 2021: Welcome Michael Appiah!
- October 2021: Leila Abdelhamid successfully defended her PhD dissertation! Congratulations Leila!
- August 2021: Jing Zhu left for biotech industry. Welcome Razan Alajoleen and Caitlin Armstrong!
- April 2021: Xavier Cabana-Puig won the AAI Trainee Abstract Award!
- March 2021: Brianna Swartwout successfully defended her PhD dissertation! Congratulations Brianna!
- February 2021: Our PNAS paper titled “Regulation of neonatal IgA production by the maternal microbiota” was featured in EurekAlert and VTNews! Welcome Ran Lu!
- January 2021: Leila Abdelhamid won the Outstanding Doctoral Student Award!












