Flow Cytometry Resource Laboratory
Flow cytometry is an essential and versatile analytical tool with numerous applications in basic research and clinical medicine. It can rapidly measure multiple characteristics of thousands of individual cells, thus quantifying different types of cells in complex, heterogeneous populations. The additional capability of cell sorting enables investigators to purify or enrich a particular cell type from a mixed population, allowing their further study.
The Flow Cytometry Laboratory maintains two locations for services: the main laboratory in the Virginia-Maryland College of Veterinary Medicine and a satellite lab in Steger Hall which is part of the Fralin Life Sciences Institute.
Our services are available to all Virginia Tech faculty, staff, and students, as well as to investigators outside the University. Current and former clients include scientists in the Colleges of Science, Agriculture & Life Sciences, Engineering, Natural Resources & Environment, Veterinary Medicine, the Virginia Tech Carilion School of Medicine and Research Institute, the Edward Via College of Osteopathic Medicine, and numerous corporate entities in the New River Valley and Roanoke areas.
Technology Highlights
What is Flow Cytometry?
- Cytometry is the measurement of chemical and/or physical characteristics of cells.
- In FLOW cytometry these measurements are made as cells in fluid suspension pass one by one through a measurement apparatus, the flow cytometer.
- Traditional flow cytometers measure fluorescence intensity and light scatter.
- Imaging cytometers measure size, shape, location and texture in addition to intensity.
- Distinct measurements are taken from each cell in a sample, giving a distribution as opposed to an average.
How does it work?
- Cells pass one at a time through a focused laser beam
- The light that emerges from each cell is collected
- The collected light is evaluated by graphical presentation
- For sorting, cells of interest are captured and purified (>98% purity in most cases)
- For imaging, pixels are tracked down the detector surface and reconstructed by the software
Equipment
The ImageStream combines the speed and objectivity of flow cytometry with the detailed imagery of microscopy to allow you to objectively and quantitatively describe biological processes based on cell size, shape, and fluorescence location, texture, and signal intensity.
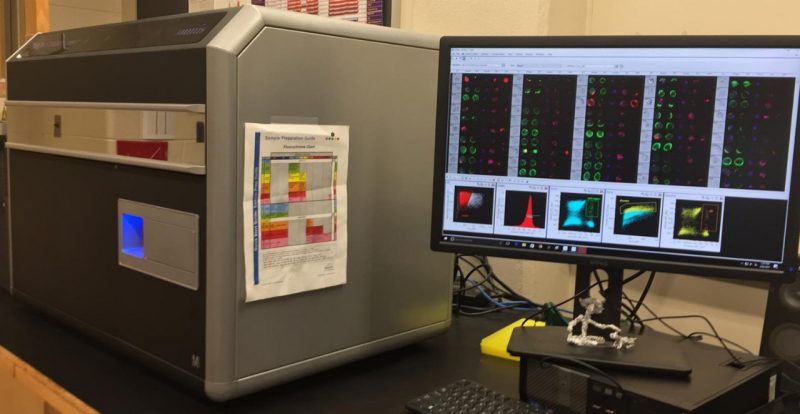
This open platform enables new methods of research and improves traditional flow and imaging based applications. It is equipped with 405nm, 488nm, and 642nm excitation sources and is able to analyze up to twelve parameters simultaneously. It has 20X, 40X, and 60X objective lenses and Enhanced Depth of Field technology. It can process several thousand cells per second.
The Fusion is a cell sorter that can also be used as an analyzer. It is equipped with 488nm, 640nm, and 405nm excitation sources and is equipped to analyze up to fifteen parameters simultaneously.

The Fusion is capable of processing up to 70,000 events per second and sorting up to four different cell populations at once. It is also equipped with an Automatic Cell Deposition Unit (ACDU) that allows sorting of cells into plates or onto slides.
Common Applications
- Apoptosis and viability
- Cell receptors
- Cytokines
- Activation molecules
- Fluorescent proteins
- Cell counting
- Cell sorting
- DNA content & cell cycle
- Proliferation
- Rare event analysis
- Cytometric bead arrays
- Microbial viability
- Intracellular markers
- Phagocytosis
Our Services
Consultation:
- Experimental Design
- Reagent Procurement
- Panel Design
- Data Presentation
- Data Analysis
- Troubleshooting
Education:
- Literature and Protocol Resources
- Lectures
- Workshops
- Seminars
- One-on-One Training
Cytometric Analysis:
- Multi-parameter immunophenotyping
- Analysis of population viability, apoptosis, cell cycle distribution, and ploidy
- Intracellular cytokines, proliferation markers, fluorescent reporter proteins
- Bead array and nanoparticle quantification
- Population morphologic characteristics
Cell sorting:
- Up to four way population sorting
- Single-cell sorting
Contacts
Melissa Makris
Supervisor
Phone: 540-231-4115
Email: mmakris@vt.edu
William Huckle
Faculty Coordinator
Phone: 540-231-3620
Email: wrhuckle@vt.edu


